chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Large volume soxhlet extraction
This is a continuation of https://www.sciencemadness.org/whisper/viewthread.php?tid=63...
There was NO HEAT EXCHANGE ISSUE. As it turned out the solvent as incorrectly documented. The published bp of 85*C was wrong. In working with
hydrocarbon mixture solvents like naptha one should always check the bp in the lab before desining the experiment. In this case the bp could have been
and subsequently was easily determined by capillary method. It turned out to be 136*C. A fresh gallon from the hardware store had a bp of 130*C. An
MSD for the same stuff indicated >240*F which translated to somewhere over 117*C. These numbers were too high for a large bowl on my hot
plate/stirrer. I used dibutyl pthalate as the bath and acetone as the solvent. Acetone boils @55-60*C. The acetone started boiling with the bath at
65-68*C and was refluxing well at 70*C. I have the hot plate at 70% power and a power cutoff set at 70*C. I feel comfortable leaving this running over
the weekend but I'd like to see one cycle to make sure the setup siphons properly. I have two large capacity soxhlets. This is the one with the
extraction tube off the side so there's lots of cooling during extraction. That requires more cycles than my other one which extracts at the vp of the
solvent. Only problem is I need an adapter for the top joint which is something like 135/120. Clamping is kind of amusing too.
[Edited on 28-8-2015 by chemrox]

"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
why create a new thread if this is a continuation ?
Not trusting the labels 100% is always a good idea.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I changed the title so I started a new thread about a successful process.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
If you want to merge and re-title, just ask a moderator.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
ELRIC
Hazard to Others
  
Posts: 244
Registered: 23-2-2015
Location: Kentucky
Member Is Offline
Mood: No Mood
|
|
Is it just me, or does the link not work for anyone else
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Which link ?
All seem OK from here.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Curious as to what the material being extracted is?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
@Bert- interesting you asked. A natural material legal in my state. A relative with a nearly hopeless case of cancer is to be the beneficiary when
it's done. The solvent is technical acetone. I had to lean the apparatus 3º away from the main body (cylinder) to get it to siphon. I since wrapped
it with Al and heating cord to maintain heat in the cylinder. Still into 300 hrs and have some color in the extract.
[Edited on 12-9-2015 by chemrox]

"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Aceton will likely dissolve all moderate molecular weight organic material (unpolymerised organic material).
As such it will dissolve chlorophyle, polyphenols and a lot of colourizers of plants but at reduced speed owing to the large quantity and particle
size.
Maybe that your target molecule is already extracted for long.
I suppose you did made a pretest on a tinier scale and with thin layer chromatography follow up?
It may be good to test various solvents with different polarities:
-Water
-Methanol or Ethanol
-Aceton
-dichloromethane
-cyclohexane/hydrocarbon
Solubility/extraction of your desired target molecule might be easier in one than in another and some perturbating stuffs might be taken out of the
path depending on the order of extraction sequence of solvents...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
For terpenoid extractions isopropyl alcohol is known to be superior to acetone, due to more selective extraction. Commercially hexane (or other
petroleum solvents) are used with excellent results.
[Edited on 12-9-2015 by careysub]
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Lighter refill gas can and use critical extraction, its quicker and better and the oil is much better purity. I think I have a link to a good resource
for this sort of thing, if I find it I will U2U you.
Its not my thing but I did look at it at one time. Basicaly you set up a plastic tube with a wire gauze at the bottom, the top has a small hole just
big enough for the tip of the gas can to fit. You packed out the plastic column leave the gauze end over a bowl and empty the entire gas can in (they
are cheap), like filling a lighter the gas comes out as a liquid.
As it passes through the material it extracts all the oils, leave it for 20 mins after the gas has run out. What your left with should just be the
required oil, cheap simple and pretty pure with no faffing,. I wish your relative well
Dont ask me, I only know enough to be dangerous
|
|
|
Sulaiman
International Hazard
    
Posts: 3696
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
suppose that someone had started a similar extraction
by putting c150g of dried plant material in methanol
producing a solution with a lot of chlorophyll present.
What would be an easy way to separate the active ingredient ?
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Extraction with an oil of your choice. Olive?
[Edited on 13-9-2015 by hyfalcon]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by Sulaiman  | suppose that someone had started a similar extraction
by putting c150g of dried plant material in methanol
producing a solution with a lot of chlorophyll present.
What would be an easy way to separate the active ingredient ? |
Ah. A plant extract "legal in your state". I can't quite make out what that might be, but I was never a Bright Young Thing.
You modern kids are soft... When we were young, we had to walk uphill the whole way to school both ways, in a blizzard,
with blue meanies constantly snapping at us, godawful slow dial up modems and NO INTERNET FOR ANYONE OUTSIDE OF DARPA!

Some have filtered such an alcoholic extract repeatedly through activated charcoall, using a Büchner funnel, side arm flask and a water powered
aspirator The chlorophyll is a large, "sticky" molecule, it will remain in the charcoal while the target substance (and a bunch of sugars and similar
crap) remain in the solvent...
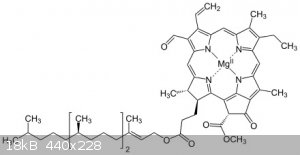
When the alcoholic extract shows no green coloration, the alcohol may be removed by vacuum distilation, the remaining extract taken up in a minimum
possible volume of diethyl ether and repeated liquid-liquid extractions done with lukewarm water, continuing until the water layer shows no color. The
ether may be distilled off and reclaimed for further use- or some have just let it evaporate away into the atmosphere.
(And I NEVER knew anyone who blew up/burned down a house doing such things. No sir, I was too busy studying and
attending the church of my communities choice, in between going to ROTC and the young Republicans on campus committee meetings to have met any such
people!)
[Edited on 13-9-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
I couldnt find a video using the plant material you describe, all I found was one using cannabis! But it should work exactly the same as the plant
stuff your working with.
https://www.youtube.com/watch?v=uw9x9MvXCf0
Chromatography would separate it I guess. But if you do it again try the method in the video, dad gave it a go once and he seemed pretty happy (for
about 2 months actually).
Dont ask me, I only know enough to be dangerous
|
|
|