dermolotov
Hazard to Others
  
Posts: 114
Registered: 13-12-2014
Location: Toronto, Canada
Member Is Offline
Mood: Free
|
|
Palladium on Carbon Hydrogenation - Working out the kinks
Designing a small home hydrogenation system and I'm not sure on how to go about this.
Let's try it with Myrecene which is a simple 3 alkene containing hydrocarbon. 5% Pd on Carbon ($30 from Alfa Aeser for 2grammes). And a hydrogenation
source.
How does one:
-Provide a good hydrogen source for 3-6 hours? Electrolysis? HCl drip on a solution of Bicarbonate?
-Work on the chambre? I'm assuming a bubbler from the hydrogen chambre into the liquid reaction vessel. Do I need it to be airtight or should the
constant bubbling suffice?
-Keep the molar catalyst ration to 10%? 20%?
-Detemine the pressure? I don't think my simple glassware can go to 3atm and I really don't want to build a nickle plated compression chambre.
-Determine temperature. I assume as low as it can be to provide maximal diatomic hydrogen dissolution?
I'm hoping to:
-Detemine the compositions of Myrecene and partial reactants after 1h, 2h, 3h, etc.
-TLC in DCM/Hexane should work. But that can be figured out later when I consult the Merck.
All help is generously appreciated!
|
|
|
dermolotov
Hazard to Others
  
Posts: 114
Registered: 13-12-2014
Location: Toronto, Canada
Member Is Offline
Mood: Free
|
|
Bump?
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
You could try a transfer hydrogenation, using ammonium formate as a hydrogen source.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I understand K-formate works better for transfer hydrogenations.. I have a paper on it somewhere. I'll edit-add when I find it.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
I think you know that won't isolate any hydrogen, try zinc or aluminum.
Quote: Originally posted by dermolotov  |
-Work on the chambre? I'm assuming a bubbler from the hydrogen chambre into the liquid reaction vessel. Do I need it to be airtight or should the
constant bubbling suffice? |
Constant bubbling should due fine, but yields wont be as good.
Also there's the explosion hazard of H2 leaking over a period of hours, but decent ventilation will take care of that.
Quote: Originally posted by dermolotov  |
-Detemine the pressure? I don't think my simple glassware can go to 3atm and I really don't want to build a nickle plated compression chambre.
|
I don't know what the pressure withstanding capabilities of your glassware has to due with measuring the pressure, but you can find both pressure
gauges and high pressure glassware on eBay or Amazon.
Why is nickel necessary? Also nickel plating is very easy if you have a nickel salt (chloride or sulfate), a power supply, a vessel, and the object
which is to plated.
Are you asking what that temperature is? Or just stating the oblivious? A thermometer might come in handy either way.
That would be a good
idea.
May I ask what this is for?
Good luck.
[EDIT] Fixed quote.
[Edited on 17-12-2014 by Molecular Manipulations]
|
|
|
dermolotov
Hazard to Others
  
Posts: 114
Registered: 13-12-2014
Location: Toronto, Canada
Member Is Offline
Mood: Free
|
|
Quote: Originally posted by Molecular Manipulations  |
1. I think you know that won't isolate any hydrogen, try zinc or aluminum.
Quote: Originally posted by dermolotov  |
-Work on the chambre? I'm assuming a bubbler from the hydrogen chambre into the liquid reaction vessel. Do I need it to be airtight or should the
constant bubbling suffice? |
2. Constant bubbling should due fine, but yields wont be as good.
Also there's the explosion hazard of H2 leaking over a period of hours, but decent ventilation will take care of that.
Quote: Originally posted by dermolotov  |
-Detemine the pressure? I don't think my simple glassware can go to 3atm and I really don't want to build a nickle plated compression chambre.
|
3. I don't know what the pressure withstanding capabilities of your glassware has to due with measuring the pressure, but you can find both pressure
gauges and high pressure glassware on eBay or Amazon.
Why is nickel necessary? Also nickel plating is very easy if you have a nickel salt (chloride or sulfate), a power supply, a vessel, and the object
which is to plated.
4. Are you asking what that temperature is? Or just stating the oblivious? A thermometer might come in handy either way.
5. That would be a
good idea.
5. May I ask what this is for?
6.Good luck. |
1. I doubt CO2 contamination would ruin the product. Bicarbs should work that way for a steady H2 and CO2 generator, no?
2. Fume hood. I have a decent set up  . How do I improve yield with or without
bubbling. Attaches into 3: . How do I improve yield with or without
bubbling. Attaches into 3:
3. If I need to build it up to 3atm, I'll construct something out of PVC tubing to withstand the pressure. but getting H2 gas in there may pose an
issue...
Nevermind Nickel. I was going to use nickel plated bomb reaction vessels but PVC should be cheaper and better.
4. I'm asking what the temperature [and pressure] should be at to maximise yield. Most articles I see is 3 atm and 0deg C.
5. Purities will have to be determined by TLC. I have no other ways of doing this. Any suggestions would be greatly appreciated. DCM/Hexane 1:1 ratio
should be good starting out.
6. Thank you. For your first post, this has been very helpful.
|
|
|
dermolotov
Hazard to Others
  
Posts: 114
Registered: 13-12-2014
Location: Toronto, Canada
Member Is Offline
Mood: Free
|
|
Cheers for the person who messaged me with the U-X (x being various transition metals. Specifically cobalt and nickel) hydrogenation method. I would
rather use a palladium on carbon as (believe it or not) raney nickel is much harder to acquire.
If anyone has any papers that includes a detailed appendix on hydrogenation, please feel free to post it here and include the footnote number. That
would be greatly appreciated as well... I, for the life of me, can't bloody remember any.
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
1. CO2 probably wont contaminate anything, but upon reaction with acids, bicarbonates release CO2 and H2O, not
H2.
NaHCO3 + HCl --> NaCl + CO2 + H2O.
2. Awesome you have a fumehood!
3. Yeah, PVC should work fine.
As for getting H2 in there, see the picture:
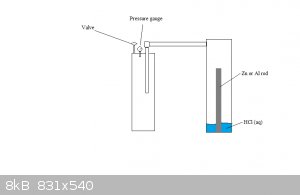
Just make sure to get all of the conections airtight.
4. Then that's what I'd try - 3 ATM can be achieved from the Al HCl reaction, and an ice bath should give you a close enough temperature.
5. No idea, try whatever seems best to you.
6. Your welcome, and thank you.
[Edited on 18-12-2014 by Molecular Manipulations]
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
http://www.sciencedirect.com/science/article/pii/S0040402001...
These techniques work quite well. Sadly, NaBH4 is difficult to acquire for some.
Most of the pertinent papers are buried in Journals that are not available on line.
Got a friendly, well stocked library near by?
|
|
|
dermolotov
Hazard to Others
  
Posts: 114
Registered: 13-12-2014
Location: Toronto, Canada
Member Is Offline
Mood: Free
|
|
Quote: Originally posted by Molecular Manipulations  | 1. CO2 probably wont contaminate anything, but upon reaction with acids, bicarbonates release CO2 and H2O, not
H2.
NaHCO3 + HCl --> NaCl + CO2 + H2O.
3.As for getting H2 in there, see the picture:
Just make sure to get all of the conections airtight.
4. Then that's what I'd try - 3 ATM can be achieved from the Al HCl reaction, and an ice bath should give you a close enough temperature.
|
Now THAT is helpful ^_^
1. Whoops. Brainfart there. looking at your diagram in (3.), zinc or aluminium with HCl seems the best option. Zinc may be the better option with a
more controlled reaction.
4. If anyone has no references, I may just need to build that hefty PVC item, now. Let's see what else is available first.
Don't need one. I have full access to web of science. 
That first article is interesting but I have to get the full one, I have to request it and go in person... On top of that, sodium borohydride is a tad
too expensive for non-catalytic reactions. Palladium seems to be best but the apparatus is just annoying to build. Recovery will be annoying but it's
only $40 for 5 grammes of 10% here.
Just need some articles with simple references to a palladium hydrogenation.
I'm thinking... a bubbler system (SATP) might be able to work if I run it for 2 or 3 times longer? I might just do this for now and report back to see
what the results are. No harm in doing it, innit?
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, if you are committed, a hydrogen generator can be purchased. At one time, they were reasonably priced on e-Bay.
Yes, NaBH4 is kinda pricey. I'm forever reading references about prices of ~40/Lb! THAT is some sort of dreamed up industrial price. Actual
prices are much higher.
|
|
|
dermolotov
Hazard to Others
  
Posts: 114
Registered: 13-12-2014
Location: Toronto, Canada
Member Is Offline
Mood: Free
|
|
Late time bump. things have been too cold nowadays to work in my shed. I might have discovered a new method for home hydrogenation.
If I could use a regular thermos soup filler. Then place everything in the solution with lye and Al metal and PdCl2. Leave over night. That should be
good enough to fully hydrogenate the substance, no?
The only issue is if you need to add acids or corrosives. Currently, I'm looking to hydrogenate a phenol ring completely and it seems I will need to
acidify it as much as possible. I might need to find some sort of teflon coated Thermos... Or should I attempt to find some PVC tubing? Would that
resist Suphuric and phenolic acid attack?
[Edited on 16-3-2015 by dermolotov]
|
|
|
Texium
|
Thread Moved
22-11-2023 at 19:18 |