uber luminal
Hazard to Others
  
Posts: 124
Registered: 25-8-2004
Member Is Offline
Mood: No Mood
|
|
Explosive chambers
Erm, I suppose this goes here, since its a piece of lab equipment.
I need to make a SAFE way of delivering molten cast iron (and a variety of other lower MP metals), into a 5 gallon bucket full of water.
Some sort of cover would be needed to shield one from flying spatter, but also in consideration would be the force of the steam generated... in an
explosive manor.
anywhere from 5-100 grams of the metal would be deposited each time.
the water bucket needs to be tall, as metals with lower Tconductivity will go further than metals with less TC.
The metal needs to be dropped from at least 30 cm, into the water.
I am pretty stumped becuase everything I devise has some danger or some flaw that I worry will kill me.
Possible materials I have available... Hole in ground, Mild Steel (i can weld),cement.
I thought maybe some of you have devised ways to set off explosives in confined spaces, and you might be able to help out.
|
|
|
Dodoman
Hazard to Self
 
Posts: 79
Registered: 2-8-2004
Member Is Offline
Mood: No Mood
|
|
I think you could place a wire net over the bucket. The net should have small openings. The wholes should vent the vapor so it won't explode. And
you don't have to worry about it melting cause it won't. I was told that jewelry manufactures in some 3rd world contries place such nets
over silver as they dry it after molding cause sometimes water gets trapped inside the pieces.
|
|
|
Twospoons
International Hazard
    
Posts: 1326
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
The risk of a steam explosion is probably not as great as you might think. I have poured 1/2 kg molten lead into a bucket with only a litre or two of
water, with no great steam eruptions. What tends to happen is non-contact boiling at first, which is fairly slow, then rapid boiling as the metal
cools sufficiently. This happens about the time the metal reaches the bottom of the bucket. The steam generated condenses again as it rises into the
cooler water above, and very little escapes the surface.
I'm assuming you're trying to make small metal beads, so will be pouring a thin, slow stream of metal in to the water.
This is not to say one shouldn't be careful, but with a large enough bucket, and a small quantity of metal I think you wont have any trouble.
Just don't stand with your face over the bucket 
[Edited on 9-11-2004 by Twospoons]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Well if the metal is hot enough, isn't the heat sufficient to split water into H2 and O2, which recombines upon cooling, making this dangerous?
Lead is obviously not a problem - but 1300 deg C molten iron maybe.
Face mask, and other gear is obviously a must! And a ladle with an extremely long handle.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
uber luminal
Hazard to Others
  
Posts: 124
Registered: 25-8-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Dodoman
I think you could place a wire net over the bucket. -- And you don't have to worry about it melting cause it won't.
|
Dodo, what do you propose I make a net out of, that is going to withstand molten iron? and I need to pour directly into the water, not through a net.
Although this does sound partialy like a good idea for around some areas where I dont want random spatter.
| Quote: | Originally posted by Twospoons
The risk of a steam explosion is probably not as great as you might think. I have poured 1/2 kg molten lead into a bucket with only a litre or two of
water, with no great steam eruptions |
The type of steam generation will depend greatly on what metal I pour. I think it depends mostly on Thermal conductivity of the material, the ammount,
and what the temp is.
If it has a high TC(thermal conduct), it will transfer heat quickly, and thus Dump it much faster. If it has a lower TC, it will take longer to
transfer the heat from the entire volume of the metal to the water. So your lead that you poured, was both low temp (not a lot of heat stored)(since
leads MP is pretty low as well) and its TC is also very low for a metal. (almost half that of iron a nearly a 7th of what Al is. So obviously you can
put Pb into water, and it will take some time before the water is heated enough that it will boil and eject steam.
Fe doesnt concern me as much as Al. While the Fe will be considerably hotter, the Al's TC is 3-4 times greater than Fe. Al will dump its heat and
dump it fast. I have had fresh welded steel fall on my arm, and the damage was just on the surface of my skin. I had molten Al fly off and hit my
other arm and transfer enough heat through the leathers into my arm and leave deep tissue damage. The contact time of both were about the same. The TC
makes all the difference.
when I do the Al there will be flying spatter, and a huge explosion. I am not sure if the explosion is just caused by the ejecting steam, or if theres
actualy enough energy in the system to break apart the water molecules for something much worse. I hope this helps any conciderations.
I will be wearing a face shield, and full body leathers. But this still wont protect me from flying cement or metal that didnt pass the stress . I
thought about pouring the metal into a seperate crucible that could then be activated remotly to pour, but I would have get the metal, run over to the
bucket of water, pour it into the other crucible, run back, activate the mechanical function, and do it all before the metal (specificaly Al) starts
to cool enough that the experiment wont work.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
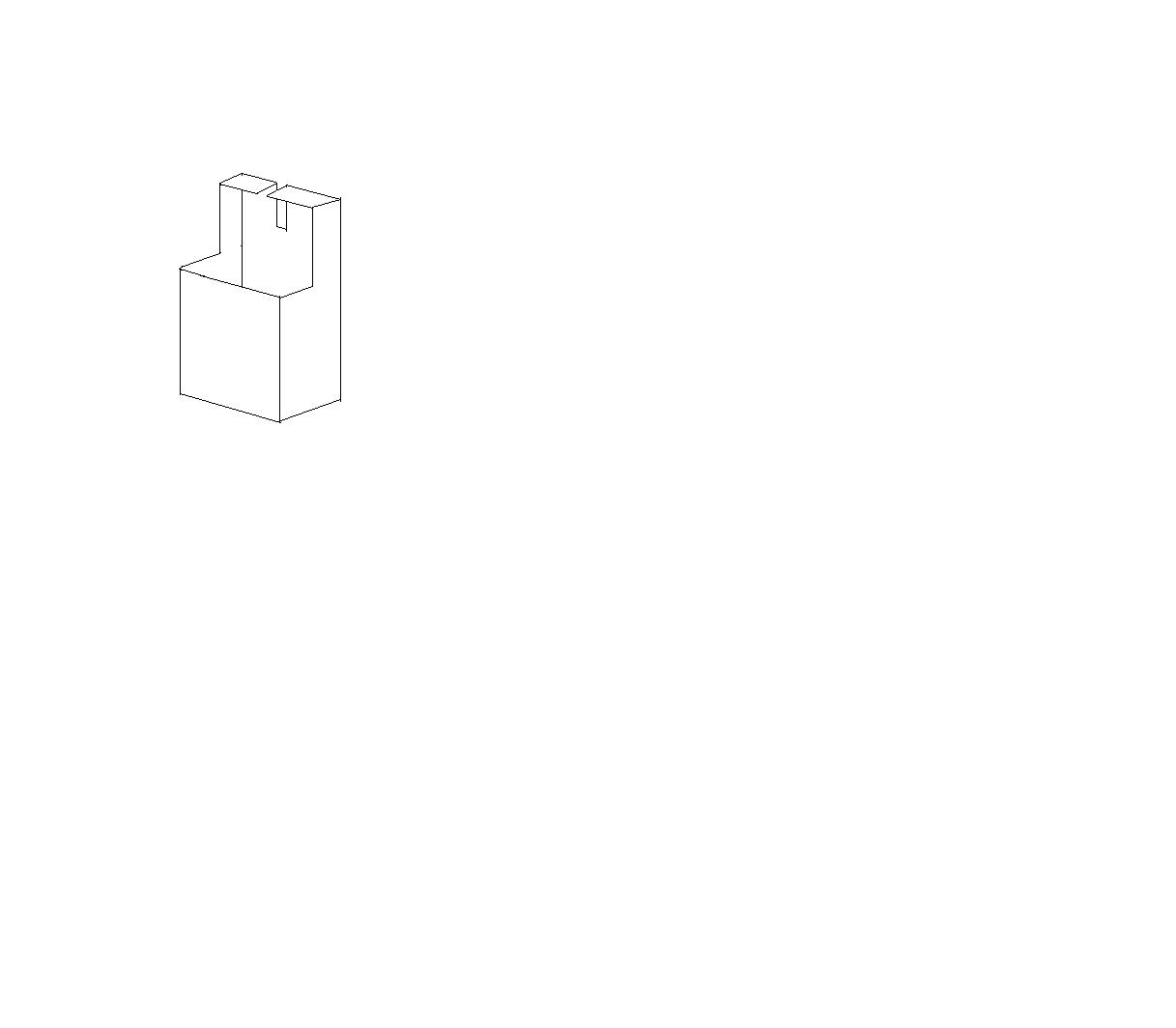
This can probably be improved upon, but here goes: Make metal container as in picture, fill with water. Take hold of crucible with molten metal with
long tongs. Get behind container, lower tong handles into slot in container. Say quick prayer and turn tongs, thus emptying the crucible into the
water.
Back of container will act as blast shield, hopefully deflecting water and/or steam and/or molten metal away from operator.
Additional protection using metal nets as well as adjusting the angle of the blast shield etc could be helpful I guess...
[Edited on 2004-11-10 by axehandle]
|
|
|
Twospoons
International Hazard
    
Posts: 1326
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
With Al you also have the issue of Al reacting with the water.
You could also try an oil quench - with a high BP oil. This would be good for reactive metals like Al. Though you will need a lid to kill the fire
afterwards 
|
|
|
Taaie-Neuskoek
Hazard to Others
  
Posts: 222
Registered: 14-5-2004
Location: Zermany
Member Is Offline
Mood: Botanical!
|
|
Dumping Al in water is risky, the water splits into 2H2 and O2, and with a little heat a rather exothermic, explosive reaction will happen.
This is why you never should try to extinguish burning Mg with water.
For oil, I don't know. It will burn at the end for sure. I don't like the idea of putting an oxidiser of 660 - 2467*C in a FEUL. Although it
looks the best option, be carefull!
Never argue with idiots, they drag you down their level and beat you with experience.
|
|
|
Dodoman
Hazard to Self
 
Posts: 79
Registered: 2-8-2004
Member Is Offline
Mood: No Mood
|
|
The net i was refering to doesn't have to stand the temp. The metal that would hit the net must have come from the water so it should have a hard
surface.
Small balls of hot iron with molten core hitting the net should bounce off it back to the bucket.
As for how to place the net this is simple. Fill the bucket to about 2/3 leave a littel space to allow the metal balls to cool a littel as it hitts
the water before hitting the net. The net should have a whole in the middle to allow pouring the iron.
As for how to pour the iron this could be tricky. I think you could gide its way in by placing a ceramic pot with a whole in the middle (the kind they
put plants in). Just make sure that it won't crack or get too hot and melt the net and fall into the water. I think you could do this by building
supports for the ceramic pot.
|
|
|
uber luminal
Hazard to Others
  
Posts: 124
Registered: 25-8-2004
Member Is Offline
Mood: No Mood
|
|
Erm... So why would adding that much heat make water break apart? then explode? I mean really, metal industries quenche metals that get even hotter
right into brine (which is mostly water anyway) and they dont have explosions?
It just doesnt make sense to me that H2O would undergo a reaction of 2H20 -> 2H + O2 then O2 + H2 --> H20
|
|
|
darkflame89
Hazard to Others
  
Posts: 255
Registered: 1-3-2004
Location: With probability 1, "somewhere" in this
Member Is Offline
Mood: No Mood
|
|
hmm.. at high enough temperatures, Mg and Al can react with water, just like sodium react with water. This action liberates hydrogen. In large enough
qunatities, the exothermic reaction between metal and water, will cause this hydrogen to react with oxygen in air, and cause and almighty explosion.
Ignis ubique latet, naturam amplectitur omnem.
|
|
|
Ium
Harmless

Posts: 24
Registered: 21-4-2004
Location: Australia
Member Is Offline
Mood: Cleaved
|
|
An O2/H2 explosion danger is most certainly present when considering reactive metals as Mg and Al. I would not even consider water for quenching when
using these materials. Even if an explosion does not occur the metal will oxidize to a considerable extent. Something to consider if purity is an
issue.
Also remember that latent heat values differ from material to material and there are reasons why water has found widespread use as an effective
coolant other than the financial benefits.
I can agree that thermal conductivity of some metals can be an issue. The higher the conductivity the shorter the timeframe for the heat transfer to
occur. The shorter the timeframe the more intense the reaction with the coolant will be. If the heat has no time to dissipate then you may get
localized hot-spots in the coolant which could present a fire or explosion hazard with water or oil.
However I have little experience in such areas as metal casting and such so I can really only offer my personal opinion.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"I need to make a SAFE way of delivering molten cast iron (and a variety of other lower MP metals), into a 5 gallon bucket full of water. "
Why?
|
|
|
uber luminal
Hazard to Others
  
Posts: 124
Registered: 25-8-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by unionised
"I need to make a SAFE way of delivering molten cast iron (and a variety of other lower MP metals), into a 5 gallon bucket full of water. "
Why? |
why not?
er rather, I need it for a MatE report. I need to provide several hard evidence/observations for a said report. By quenching the different molten
metals, they do a variety of different things. I can provide evidence for thermal conductivity etc. by the way the metals freeze in the water. Its an
oddball experiment but it has worked thus far for Os-Pewter, zinc, tin and lead.
|
|
|