axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
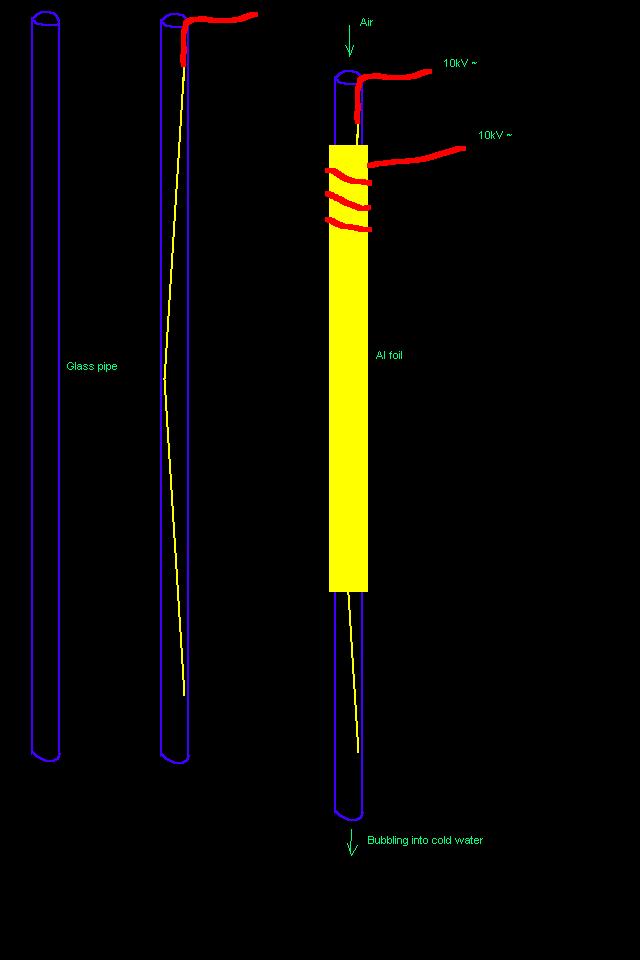
H2O2 by ozone -- The slow method.
By my calculations, 0.24m<SUP>3</SUP> of ozone should suffice to convert 1dm<SUP>3</SUP> of water to 35%
H<SUB>2</SUB>O<SUB>2</SUB> solution, given 100% efficiency.
I have no idea of how efficient the ozone generator I just invented and whipped together is (attaching a drawing), but this is probably going to take
a while. The bottle contains 0.5dm<SUP>3</SUP> of water.
I'm feeding the thing with 9kV AC, BTW.
Comments?
Notice the blue light from the corona discharge:

EDIT: *cough* *cough* No, it's not 100% efficient. I think I have to solve the problem of reacting tail ozone before running this for long.
[Edited on 2004-10-1 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
Unless you use pure oxygen (vs air in the room), how do you know that you don't get HNO3 instead of H2O2?
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
It's not a plasma discharge but a corona discharge. No arc -- no NO<SUB>2</SUB>.
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
FrankRizzo
Hazard to Others
  
Posts: 204
Registered: 9-2-2004
Member Is Offline
Mood: No Mood
|
|
It appears that corona discharge (if the energy involved is high enough) will produce NO<sub>2</sub>.
http://www.fysik.uu.se/doktorand/doktoranddagen/Abstracts/Ma...
NOx production in spark and corona discharges
Mahbubur Rahman, Nils Rehbein, Vernon Cooray
Division for Electricity and Lightning Research, Department of Materials Science
The Ångström Laboratory, Uppsala University, Uppsala
Abstract
This paper presents an experimental study on the production of NO and NO2 as a function of electrical energy in
laboratory sparks and corona. The results show that both the NO and NOx yield increase almost linearly with the
energy per unit length of the discharge. From the data, it is estimated that the spark discharges with energy in the
range of 0.5-2.0J....
[Edited on 2-10-2004 by FrankRizzo]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I would not do it with ordinary air - only with pure O2. Besides, with air, nitrogen oxides are thermodynamically favored over O3 production.
John W.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Oh, please, people have been using ozonizers with air for a very long time. Of course one can get higher and faster yields with O2, but the O3 yield
is still very much higher than the NxOx yield. Given low voltage/residence time, of course.
http://www.orgsyn.org/orgsyn/prep.asp?prep=CV3P0673
axehandle, exactly how are you planning to get H2O2 from this?
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
| Quote: |
axehandle, exactly how are you planning to get H2O2 from this?
|
By bubbling the O<SUB>3</SUB> through cold water. Should work, according to something I read somewhere.
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
rikkitikkitavi
Hazard to Others
  
Posts: 192
Registered: 17-6-2002
Member Is Offline
Mood: No Mood
|
|
but first you will ozidize any organic matter so it would be best to seal the vessel so no dust can enter it. Also, have you considered the fact that
ozone is very toxic.
also, you should use deionized water, otherwise the H2O2 will decompose due to dissolved metals e t c.
Fortunately the odour threashold is about a magnitude lower than the lowest level where ozone starts to have physiological effect. But perhaps you
will not have a winter cold when this is operated.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
I agree that bubbling ozone into water should form trace amounts of hydrogen peroxide, but it is also my understanding that ozone destroys hydrogen
peroxide.
|
|
|
sarcosuchus
Harmless

Posts: 25
Registered: 16-9-2004
Location: in server hell
Member Is Offline
Mood: sleepy
|
|
this is something i do know from hands on research your going to get hno3 unless you use pure O2 and pure h20 (see my post on plasma cutters).....real
dumb thought but if you burned H2 with O3 in argon with a excess of O3 would it favor H2O2 if it was cooled rapidly say 0.1 sec ???
famous last words\"hold my beer and watch this\"
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
If you use dry air you will get a proportion of nitrogen pentoxide, this can be small compaired to the amount of ozone though.
An ordinary oxyhydrogen blowpipe flame set against a piece of ice, the water run off will contain hydrogen peroxide. Enough for a chemical test at
least. I doubt when it comes to combustion ozone will make much difference unless it is in great excess and the heat is just used to decompose it
with water vapour. I dont see any of these methods producing useful amounts of peroxide.
|
|
|
Hermes_Trismegistus
National Hazard
   
Posts: 602
Registered: 27-11-2003
Location: Greece, Ancient
Member Is Offline
Mood: conformation:ga
|
|
Absorbtion train
recently read about something called an absorbtion train that old-timey chemists used when trying to dissolve gasses in liquid, It simply consists of
jumping the gasses that bubble off the top of one flask and bubbling them through another flask.
actually.....

Arguing on the internet is like running in the special olympics; even if you win: you\'re still retarded.
|
|
|
lefty
Harmless

Posts: 9
Registered: 9-11-2004
Member Is Offline
Mood: Blevy
|
|
There's a better way to build an ozone generator.
I used a leibig condenser (but any glass tube would do) with aluminum foil wrapped around the outside and a steel rod through the column in the
middle.
Hooked up to a 15,000 V step-up transformer, the whole thing glowed purple.
I used an aquarium pump to force air into the cooling jacket of the condenser.
You should know that using air, only 1 to 2 % by volume of ozone is generated. If you use pure O2, you can get up to 3 or 4% according to the
literature I've read on it.
I've done a lot of research and experimentation with Ozone, I'm not sure if it will cause the conversion of water to H2O2 - don't know.
I do know that it will destroy organic compounds dissolved in the water and oxidize/change/destroy other dissolved solids (nitrates for example)
It is used as a substitute for chlorination in some water treatment facilities. I toured one once. The ozone generators are glass tubes about 3 to 4
inches in diameter with an Ag film on them. The spark gap in these industrial generators is on the order of 1 millimeter.
Just in case you weren't aware, ozone can destroy anything rubber in your house, so watch your refrigerator seals for example if you're
experimenting with Ozone. It also damages the cilia in your lungs - can be pretty nasty stuff.
Better to remain silent and be thought a fool than to speak up and remove all doubt.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
This method won't work. Ozone can not be combined with H2O to obtain H2O2. Gmelin mentions no H2O2 formation occurs between O3 and H2O. Ozone and H2O2
also decompose each other: O3 + H2O2 = 2 O2 + H2O. Where interaction of O3 and H2O2 is known to form dihydrogen trioxide (H2O3), which decomposes
rapidly, a bit more about the intermediates here.
I've also tried it. Having bubbled ozonized air into regular water for about 1-2 hrs time, then adding the resulting solution to KI gave no
brown/yellow discoloration (no H2O2). Even in very dilute amounts H2O2 still discolors KI.
Another method to H2O2 is using direct current against water, here is a paper which describes using DC on water utilizing a carbon felt cathode and RuO2 coated titanium anode to form H2O2, though apparently
in low concentration, around to or a bit lower in concentration than when forming H2O2 from ice and an oxytorch flame.
|
|
|
Saber
alias Picric-A
 
Posts: 56
Registered: 6-9-2008
Location: England
Member Is Offline
Mood: vry vry bad mood
|
|
For ozone generation, yields are greatly increased by using a negative corona discharge.
Keep temps low, to maximise yield, however i advise passing through a calcium/sodium peroxide washing tube to remove NOx.
I have used O3 in the past to oxidise SO2 to SO3, until i built my glass contact proccess... 
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Being able to prepare oleum by the contact process must be a real buzz---what catalyst do you use?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Yes please post complete details for us! It would be a remarkable step for some of us to be able to make conc sulfuric and oleum! I intend to do this
myself at some point but havent got the time or money at the moment  How do you
combust the sulfur? In the glass? Oxygen/Air inlet? What kind of glass? How do you
combust the sulfur? In the glass? Oxygen/Air inlet? What kind of glass?
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
With a professional type ozonizer there might not be any nitrogen oxides, at least not by me (moist litmus paper won't turn red after being held in
the stream for some time, no NO2 odor either). Also according to some literature O3 won't react with dry SO2. Liquid O3 and SO2 don't react either.
[Edited on 8-5-2009 by Formatik]
|
|
|
Saber
alias Picric-A
 
Posts: 56
Registered: 6-9-2008
Location: England
Member Is Offline
Mood: vry vry bad mood
|
|
Preparing Oleum on tap is extremely useful however there is only so much oleum you can use, so I never make more than a liter a day, if that,
My catalyst of choice is supported V2O5, obtained from purifying and activating pottery grade V2O5.
My sulfur burner is the only non glass item in it. It is manufactured from Stainless steel and I have found it resists attack perfectly. The rest is
made of Borosilicate glass. I chose Boro because it is easy to work with.
@ Formatic- simultaneously bubbling a mix of SO2 and O3 through cold water produces H2SO4, proven fact, The only thin I have against this is expense
and the fact it produces high sulfur oxoacids.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Quote: Originally posted by Formatik  | | With a professional type ozonizer there might not be any nitrogen oxides, at least not by me (moist litmus paper won't turn red after being held in
the stream for some time, no NO2 odor either). Also according to some literature O3 won't react with dry SO2. Liquid O3 and SO2 don't react either.
|
AFAIK, all airfed ozonisers produce some NOx in moist air---moisture raises conductance (H2CO3) of the air, producing hotter microdischarges to the
point where (some) N2 bonds are broken.
Some pro units have an integral air-dryer to overcome the problem. . .
As to O3/SO2, I think the oxidation is catalysed by a trace of H2O and possibly a high temperature.
|
|
|
bquirky
Hazard to Others
  
Posts: 316
Registered: 22-10-2008
Location: Perth Western Australia
Member Is Offline
Mood: No Mood
|
|
chould you not dry normal air first through sulfuric acid or sucking it through a domestic frezer ?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
To me even that's an understatement. . .
If you could offer it for sale everyone on this board would be potential customers.
But fuck it, we live in a chemophobic world.
Any chance that you might post a few pics. . .
|
|
|
Saber
alias Picric-A
 
Posts: 56
Registered: 6-9-2008
Location: England
Member Is Offline
Mood: vry vry bad mood
|
|
At current i will not be able to upload any pictures as i do not own a camera, I will though asap.
Like you said though Hissingnoise, i wouldnt dare post Oleum, unless you pay ridiculous Hazmat fees,
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
We're looking forward to seeing them, Saber---even cellphone pics. . .
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Quote: Originally posted by Saber  | @ Formatic- simultaneously bubbling a mix of SO2 and O3 through cold water produces H2SO4, proven fact, The only thin I have against this is expense
and the fact it produces high sulfur oxoacids.
|
It needs the H2O for the reaction I know this that's why I said dry SO2. Thus, SO3 isn't a product.
|
|
|