| Pages:
1
2 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
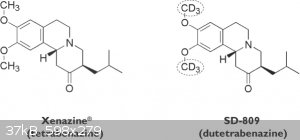
Recently, the phase III clinical trials of SD-809, a d6 version of the drug tetrabenazine, were completed and the drug will be applied for
approval. This is an interesting example of how deuteration can dramatically reduce the metabolism of a drug, while all the other activities remain
the same.
Indeed, the smell is currently believed to be connected with the vibrational modes of a molecule. The D-O bond does not have the same vibrational
frequency as the H-O bond. It is therefore not unusual that people can distinguish the taste and that rats recognize D2O by smell and taste
(as described in the Proc. Soc. Exp. Biol. Med. article linked above.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
The dizziness is usually attributed to a small difference in density in the fluid in two separate compartments in the vestibular organ, which is
important for the sense of balance. The difference arises because the rate of diffusion of D<sub>2</sub>O and H<sub>2</sub>O
into these two compartments is different. The effect is also observed upon ingesting glycerol or ethanol.
So, D<sub>2</sub>O seems like a very expensive means to become drunk really briefly.
[Edited on 29-8-2015 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
| Pages:
1
2 |