BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Electrochemical fluorine production.
The electrochemical production of fluorine is the most time tested method to produce this monster of an element. Yesterday while looking through my
library's collection of 'Inorganic Syntheses' I ran across a whole section of laboratory fluorine production apparatuses, complete with
diagrams, and other real world figures. One even said it could easily be built for under $10 (but the book was from 1964 so maybe it's closer to
$30 now). Nevertheless one design caught my eye, running off the widely available potassium bifluoride today I share this with you.
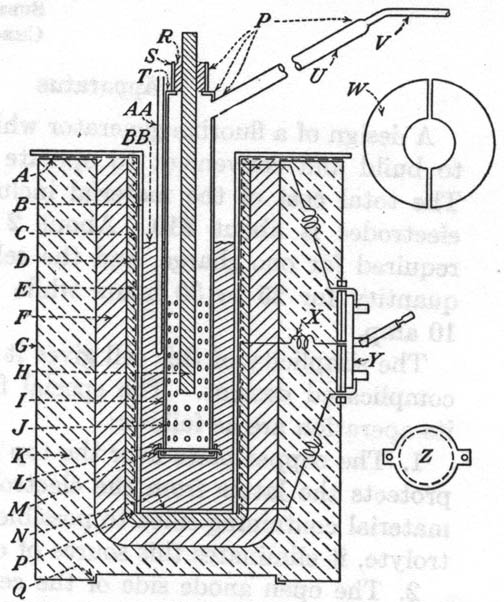
| Quote: | | A, copper plate 1/8 in. thick of sufficient size to overlap the furnace. It is securely silver soldered to the well. The negative
lead from the direct-current source is connected to this plate by means of a battery clip; B, well, made of copper tubing 12 in.
long, 3 in. i.d., and a wall thickness of 1/8 in.; C, asbestos paper wrapping; D, nichrome wire heating element, 50
ft. of No. 18; E, alundum cement coating about 1/4 in. thick; F, coating of a mixture of fire clay and sand about 1
in. thick. This gives rigidity and permanence to the heating unit and is inexpensive; G, sheet-metal can, usual type of container
for chemicals; H, electrode, graphite rod 12 in. long and 1/2 in. in diameter, made of a special mix with a minimum of silicon
binder. The positive lead from the direct-current source is connected to the top of this electrode by means of a battery clip; I,
diaphragm, made of copper tubing 12 in. long, 1 3/4 in. in diameter, and with a wall thickness 1/16 in.; J, holes drilled in
diaphragm, 1/8 in. in diameter; K, magnesia or asbestos refactory packing; L, bottom of diaphragm made of 1/16-in.
copper sheet; M, copper wire for connecting the bottom to diaphragm; N, bottom of well, made of 1/8-in. copper
sheet; P, joints made with hard-silver solder; Q, press lid of sheet-metal can; R, Portland cement
seal; S, seal tube made of copper tubing 1 1/4 in. long, 1 in. in diameter, and 1/16-in. wall size. It is convenient to support the
diaphragm by means of a clam on this tube; T, copper disc with 3/4-in. center hole; U, fluorine exit tube, 1/2 in.
in diameter and 12 in. long; V, copper tube 1/4 in. in diameter. W, cover for cell when not in use, made of
1/16-in. copper plate in two parts with center hole large enough to fit around diaphragm; X, center lead from heating element;
Y, double-throw double-pole switch, so connected as to enable the two halves of the heating element to be placed in either parallel
series. The series connection is for the regular operation of the cell; the parallel, for raising the temperature. Transite boards inside and
outside the metal can form the base upon which the switch is fastended; Z, detail construction of bottom of diaphram;
AA, thermometer well, a copper tube sealed at the bottom. It is convenient to have this only temporarily fastened to diaphragm with
copper wire or better supported from a ring stand. A thermocouple is more convenient to use then a mercury thermometer. If the later is used, it
should be covered with a film of heavy oil; BB, electrolyte. |
This is the high temperature version it runs between 210 C and 290 C, when the temperature starts to rise to the upper end adding more HF is necessary
but for the initial charge the composition is HF:KF therefore potassium bifluoride. When not in use the cell can remain cool with the electrolyte
exposed to the atmosphere without any complications.
The cell runs between 10 and 20 V but if the cell polarizes this can raise to more then 50 V. Normal operation is at 10 A.
Fluorine has always seems scary to me, but building one of these to sit around sounds like fun.
Any thoughts?
[Tried searching around for this topic first, only found chemical production of fluorine, but you know how our search engine here is...]
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
There are some things that don't seem so practical with this setup...the big one being the use of asbestos. I don't know how easy it is to
find these days as I've never bothered to look, but I don't imagine it would be very easy.
Also, am I misunderstanding something or will the resultant, hot Fluorine gas be led out through a copper tube  . Is Fluorine not very reactive against copper?? I always thought only
Nickel-lined vessels could resist Fluorine...but if not, this would be very interesting and useful. Surely we could adapt this to a new, modified
cell that does not use asbestos. . Is Fluorine not very reactive against copper?? I always thought only
Nickel-lined vessels could resist Fluorine...but if not, this would be very interesting and useful. Surely we could adapt this to a new, modified
cell that does not use asbestos.
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Fluorine lead though copper will develop a copper fluoride coating on it that will resist further attack. Nickel and even monel metal are not immune
to fluorine and also rely on this coating (I believe). It is very adherent but you can't bend the tubing if it is working at full capacity as it
will create a 'Hot Spot' as the coating flakes off internally and can cause a rupture.
But we would need a substitute for that asbestos paper
C. Hummmmm what is used to substitute asbestos these days.
One other interesting thing I was thinking about. Glass in this setup is a no-no but is not necessary as the hydrogen producing side of the cell is
seperte from the fluorine producing compartment and the electrolyte is therefore exposed allowing for monitoring of it. But there is a way to look
into the fluorine side. Back when I used to go to 'rock shops' for minerals they always sold CaF2 in either blocks that were clear (like
glass) or thin shards that were clear. Therefore you could make a window into your vessel. 
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
For asbestos, look for chrysolite instead, it's the same thing (one was used for the media scare the other for the industrial use  ) )
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
I may be reading the diagram incorrectly, but isn't the asbestos just used for insulation? Wouldn't any nonconducting temp. resistant paper
or substance work?
My idea is to use perlite, one of my new favorite substances. Perhaps the perlite could be ground up, and mixed with a clay or cement binder to form a
paper, Ok , I'm rambling.
BromicAcid, how are you going to store the fluorine? Copper pipe chamber? Could a flask be made of CaF2? Maybe I'm just too tired right now,
but a block of CaF2 could be hollowed out with a lathe, and then a CaF2 stopper could be made, you get the point.
It would be worth the extra effort just to show people the nearly invisible fluorine! 
|
|
|
I am a fish
undersea enforcer
   
Posts: 600
Registered: 16-1-2003
Location: Bath, United Kingdom
Member Is Offline
Mood: Ichthyoidal
|
|
| Quote: | Originally posted by BromicAcid
Fluorine lead though copper will develop a copper fluoride coating on it that will resist further attack. |
You will need to ensure that the reagent and apparatus are extremely dry, as water will disrupt this protection.
1f `/0u (4|\\| |234d 7|-|15, `/0u |234||`/ |\\|33d 70 937 0u7 /\\/\\0|23.
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
| Quote: |
BromicAcid, how are you going to store the fluorine?
|
IF I were to make this cell, and not just use it when I needed fluorine. I would store it in a chemical compound. Buy a tank of
Xenon and react it with that to make Xenon Difluoride, or maybe react with Manganese or Cobalt whose higher oxidation states with fluorine decompose
when heated releasing free fluorine. I would not try to store a pressurized cylinder that was home-aid, and a non-pressurized cylinder simply
wouldn't hold enough to be reasonably useful.
I'm not sure what the asbestos is for, in the one thing that calls for it and lists no substitutes it is a wrap, maybe a barrier of last resort
for if the electrolyte eats through the copper pipe?
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
What! No 50 L 10 bar homebrew bottles full of fluorine under your bed!?
Storage as a salt/compound sounds safer of course, but a tank of xenon might be expensive!
As a salt, some of the fluorine would be lost when the metal gets to its lowest feasible oxidation state, right? 
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I've been sort of back on researching fluorine again and I was wondering if anyone had any of the original papers by Moissan on this subject, or
a sketch of his original cell?
Also, I was reading about some of the early cells that used a graphite cell instead of a copper, nickel, or platnum body, where the cell body acted as
the cathode, no wonder some of them exploded. Also, one more name for asbestos is non-fiberous silica, which has a brand name that is'nt being
strongly heald in my memory right now.
Edit: Patent's 1484734 and 1484733 are decent looking fluorine cells.
[Edited on 10/6/2004 by BromicAcid]
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
Materials changes...
Hallo to all,
as knowledge, fluorine reacts with almost all that don't already contain it.
In this case, it would be best to substitute some parts of the equipment previously sayed with teflon parts, which doesn't react with
fluorine....
I hope to be useful
Thanks for the collaboration
|
|
|
JustMe
Hazard to Others
  
Posts: 111
Registered: 7-8-2003
Member Is Offline
Mood: No Mood
|
|
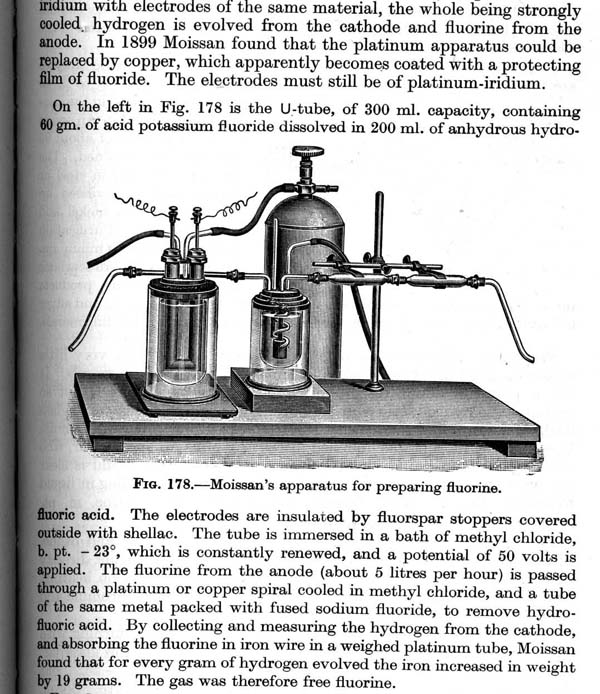
Bromic Acid,
Well, actually I have a chemistry book with a picture (looks like a detailed etching) of Moissan's apparatus for preparing fluorine, and a
description of it's construction and use.
What I don't know is how to "attach" a scan to a posting. Hope it dosn't require a link to a web page because I don't have
one. But if you can tell me how to prepare and attach such a scan, I'd be happy to do it for you! Let me know.
The actual book is an oldie but a goodie, "A Text-Book of Inorganic Chemistry" by Partington, Sixth Edition, 1961. A "Classic" in
chemistry. Very detailed, but old.
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
JustMe, you scan in the picture then when you make a post there is a button at the bottom with an empty space next to it that says attach. It uploads
the picture to the sciencemadness webspace and from there it makes it viewable as a link.
Kazaa, teflon is good for some of the vessel, however it is not good for most things. Mostly it is bad for high temperatures, however for the
preparation I have for perbromic acid, involving leading fluorine gas into the bromate solution, it warns that the teflon tubing will catch on fire if
left stationary and warns against leaving it unattended. However that might have something to do with insitu oxidizing agents the fluorine might make
with water.
Rather then teflon, Portland cement becomes quite impervious to fluorine gas after being exposed to it, forming a surface calcium fluoride coating
that is highly inert.
|
|
|
JustMe
Hazard to Others
  
Posts: 111
Registered: 7-8-2003
Member Is Offline
Mood: No Mood
|
|
Bromic Acid,
OK, hope this goes well, attaching a scan of said apparatus (and if you are interested, the book also has a picture of a more modern setup
..."using molten KHF2, Acheson graphite rods as electrodes insultated with Bakelite cement stoppers and a current of 5 amps at 12 volts."
|
|
|
JustMe
Hazard to Others
  
Posts: 111
Registered: 7-8-2003
Member Is Offline
Mood: No Mood
|
|
Hm, I'll try again, made image smaller.
|
|
|
JustMe
Hazard to Others
  
Posts: 111
Registered: 7-8-2003
Member Is Offline
Mood: No Mood
|
|
So, do y'think you could make some fluorite stoppers? 
Good luck in your endeavors (to one who has tackled another monster: phosphorus!)
|
|
|