plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
Present your electrolytic cell! (Picture intensive)
I know that many member have chlorate/perchlorate/bromates/etc cell here on sciencemadness. Sometime I want to build a new cell but I do not have any
inspiration for the design, it may happen to other also. In this thread you post picture of your cell/power supply and explain it's specs, How you run
it and general information about the cell. I have also attempted to look on website for inspiration but it seam that many electrolytic cells website
are broken...
Hypothetical Exemple:
(Picture)
Cell type: Chlorate
Anode: Carbon rod from 6V lantern battery
Cathode: a Stainless spoon I bough at the dollar store
Cell capacity: 150ml
Cell container composition: Borosilicate Glass beaker
Voltage: 5V but may vary from 4 to 6
Amperage: 1amp
Electrolyte: Sodium chloride saturated sol.
The Cell environment: It is outside so I did not add a vent tube.
Cell efficiency: 20%
I made this cell because I wanted chlorate ''now''. I run it with NaCl since after a run I can simply filter the solution and add saturated potassium
chloride solution to get quite pure potassium chlorate. I don't want to run this thing inside since it is quite smelly, I did not have
dichromate/chromate on hand so I run.....
Actually I'm looking for a new concept of cell for a platinum anode in the objective of electrolysis experiment.
[Edited on 27-6-2012 by plante1999]
I never asked for this.
|
|
|
Diablo
Hazard to Others
  
Posts: 113
Registered: 17-9-2011
Member Is Offline
Mood: Autodidactic
|
|
A simple indoor low volume hypochlorite cell in a jar, not yet constructed (probably will do tomorrow)
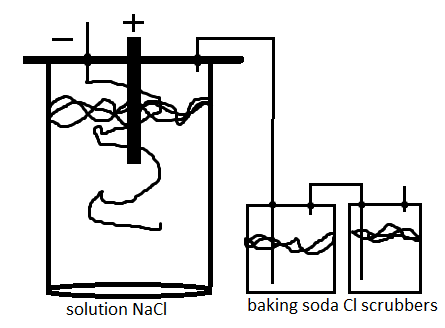
Electrolyte NaCl
Cl scrubbers have a solution of sodium bicarbonate
cell will have to be kept cool Im running it for some NaOCl for the haloform reaction.
Edit: anode 6v battery carbon, cathode steel
[Edited on 27-6-2012 by Diablo]
|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|



Cell type: General
Anode: 1/4inch Carbon rod/ 0.5mm platinum wire by 7cm
Cathode: titanium mesh 13 square cm
Cell capacity: 700ml
Cell container composition: polypropylene food container
Voltage: Depend on the solution to electrolise
Amperage: Max 0.5amp
Electrolyte epend on what I want to make epend on what I want to make
The Cell environment: Inside
I took my old cell to make this one. I changed the cathode for a smaller one and added a 1/4 inch rubber thing so I can change the anode. At this time
the anode is carbon but when I will receive my platinum wire I will use the glass pippet to make a platinum anode. I will make many type of
electrolytic synthesis with the cell, like persulphate/iodate/perborate etc... I added a vent pipe with a 25feet plastic tube to make the exhaust go
outside for corrosion prevention in the garage. I used a large container for prevention of heating when the cell is running.
Feel free to comment.
I never asked for this.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|





Dual cathode, swappable anode, MMO or Pt, using appropriate potassium salts. I don't subject Pt to chlorides if possible.
I have since added a motorized stirrer using a PTFE rod and Ti paddle. Note the PTFE solenoid valve for HCl additions. I got rifd of the ridiculous
overkill of a dosing pump, and will go with timed gravity feed in the future. I made all these mods after I started PbO2 work, and have yet to do a
run using these mods, but I think they'll work OK.

|
|
|
Broken Gears
Hazard to Self
 
Posts: 96
Registered: 7-8-2005
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Cell type: Castner Tiegel
Anode: Copper
Cathode: large iron ring
Cell capacity: 2400ml
Cell container stainless steel
Heat source: old heating element from a injection molding machine
Voltage: ?
Amperage: ?
Never had a run at it. As you probably can tell, Im more the machinist than the chemist. The idea was to produce large amounts of sodium continuously.


|
|
|
plante1999
International Hazard
    
Posts: 1936
Registered: 27-12-2010
Member Is Offline
Mood: Mad as a hatter
|
|
@Swede, this cell seem a little overkill for chlorate production on amateur scale, did you sell some of your chlorate/perchlorate?
@Broken gears Are you planing to run it someday?
I never asked for this.
|
|
|
Broken Gears
Hazard to Self
 
Posts: 96
Registered: 7-8-2005
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Yeah someday. I know that there are 2 well discussed subjects on that matter in the Technochemistry forum, but I haven't read it all and I still kinda
uncertain about the power supply and settings. I'll be sure to let you know and post some pics on the day of launch 
|
|
|
99chemicals
Hazard to Others
  
Posts: 174
Registered: 24-3-2012
Location: In the Octet
Member Is Offline
Mood: No Mood
|
|
My electrolytic cell is a beaker with a 12 volt 1 amp dc transformer. I used it before for Cobalt oxide production and iron oxide production with a
carbon cathode.
In those I used sodium bicarb as a electrolyte.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
@plante1999 - My cell is a bit of overkill simply because I enjoy doing it so much. A really active pyrotechnician can go through many kilos of
(per)chlorate in no time at all, but I have stopped doing pyrotechnics and simply got sucked into this process, purely for the challenge.
The cell pictured was an attempt at a 2-cell pumped system that was supposed to continually produce. The hot liquor would be pumped from a smaller
electrode chamber cell into that larger vat for collection... the vat itself did not have electrodes. Because I insisted on working with potassium
salts, the hot, saturated liquor, being pumped from the electrode chamber, crystallized far too quickly and jammed the whole thing solid. I damn
near boiled the EC dry overnight; the cathodes warped like bananas.


In the end, I recognized it was grossly over-engineered, and backed off a bit. Made it much simpler.
I never sold anything, and don't plan to. 
[Edited on 2-7-2012 by Swede]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Broken Gears, fire that puppy up! I like it, very neat and well constructed.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Amazing cell's people seriously. Swede if you could explain how you fixated the PTFE rod to the motor shaft I would be very appreciative!
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
The PTFE shaft was drilled on the end for a snug fit vs. the motor shaft. The motor shaft has a flat, and the PTFE rod was of a diameter large enough
to drill and tap for a pair of set screws.
The PTFE shaft rides in a bushing also of PTFE, with adequate clearance. I'd rather have some salt creep there than a galled shaft.
At the end of the shaft, I used a titanium bolt to attach a sheet Ti paddle, approx 75mm long x 20mm wide. I used a pair of pliers to bend the
paddle. It really moves the liquid! I positioned it so that it is near the top of the cell and pushes liquor downwards strongly. Hopefully it'll
remain clear of crystals. If I was doing sodium salts, I'd reverse that, and have the paddle pull liquor from the very bottom and bring it up.
Bubblers for potassium salts are worthless. Every one I've tried have jammed with xtals as the cool air enters the hot liquor. I suppose if a
bubbler was connected to a decently sized PVC pipe that was immersed, it'd do better, given the much wider bore. But even a 3/8" PTFE tubing has
jammed solid on me in the past.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Ah, I need to clarify on that shaft. What I described was incorrect. What you have there is a coupler tube made of stainless steel. The coupler
tube slips onto the motor shaft, and is held in place with set screws in the tube, which is thick-walled.
The solid PTFE shaft slips inside the coupler, and a single stainless cap screw goes completely through the PTFE shaft and is threaded on the far side
of the coupler. I also ran the motor in a test run in water, then grabbed the shaft and applied a load with my fingers, and noted the current spike
from something like 800 mA to 2.0A... then added a 2 amp slo-blo fuse in the circuit so that if the stirrer loads excessively, it'll blow the fuse.
Since you have a mini-lathe, such a system would be simple to replicate.
|
|
|
497
National Hazard
   
Posts: 778
Registered: 6-10-2007
Member Is Offline
Mood: HSbF6
|
|
Very nice cells!! Keep up the awesome work!
I found this method of taking a normal airlift pump and turning it in to a super cheap variable dosing pump. Though it might be useful for all kinds
of things. Didn't want to start a thread, and this was one of the few threads I found talking about dosing pumps, so I'll put it here. Called a Geyser
Pump.
http://www.nesc.wvu.edu/nsfc/Articles/SFQ/SFQw02_web/SFQw02_...
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I like the notion of using air to move fluids external to a cell, INTO the cell. Even a cheap aquarium air pump has the guts to displace fluid up and
out of a sealed container, but the challenge always remains accurate dosing when using a makeshift system.
I like peristaltic pumps as well. They can be found reasonably cheaply on eBay.
|
|
|