| Pages:
1
2 |
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Well, lithium has the most negative reduction potential at STP, but in a thermite reaction there are lots of things that can shift the balance. The
high stability of MgO and Al2O3 could shift the reaction, them being refractory oxides with high lattice energies.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Online
Mood: nucleophilic
|
|
I was going by what bromic posted:
"Reduction of LiOH or Li2CO3 or LiCl with magnesium as stated in Complete Treatise on Inorganic Chemistry is explosive but 40% magnesium oxide can be
added to moderate the reaction. Information relating to chemical reduction by Mg or Al can be found if you see Warren, Chem. News, 1896, 74, 6, and
U.S.P. 2028390. Lithium salts such as LiNO3, Li2CO3, and LiOH can be reduced with carbon or metal carbides or even calcium at high temperatures.
Zirconium is an effective reducing agent for nearly any alkali metal that is combined with oxygen, it will reduce sulfates and phosphates in a self
sustained reaction."
And to clarify, yes, I will be doing this under deoxidized argon but perhaps I will use alumina for the reaction vessel.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Yes, you're right. I forgot that standard potentials are only valid at STP.
|
|
|
BromicAcid
International Hazard
    
Posts: 3244
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Okay, going off what I posted earlier about lithium chloride electrolysis, 10 grams of lithium chloride and 11 grams of potassium chloride were placed
into a steel crucible. The two components were heated with a propane torch and fairly rapidly the bottom of the mixture liquefied and within a few
minutes it was entirely liquid. It was at this time that the crucible was connected to the power supply and made into the anode of the circuit and a
nickel rod was made into the cathode.

So, immediately the nickel wire coated itself in the melt and refused to perform electrolysis considering it was much colder then the surroundings so
I scraped it on the bottom of the crucible until it made a connection (evident by the amp meter shooting up past 15) and then raised it up slightly
whereupon bubbling became very rapid (the usual spray of bubbles). Nothing much happened at first, likely water was being electrolyzed as is the case
with most of these melts and it is also mentioned as a possibility in the outline of this technique mentioned earlier.
Finally the area surrounding the cathode quickly glowed red and the amps being consumed shot up past 15 and popped the fuse in the power supply.
Success, lithium likely formed in the shallow space between the anodic crucible and the cathode right above it. After the circuit breaker kicked back
in I tried again, just the slightest contact of the cathode with the surface of the melt instantly rose the amps consumed past 10 so the melt is
highly conductive. As soon as the wire touched the surface a red glow would spread out and occasionally pop sending out glowing embers or fire would
sputter up (see the glow in the picture above).
This was just a proof of concept after all. Although I did expect it to work. There is great promise in this technique in that temperature control
is not as necessary as it is in electrolysis using the metal hydroxide and I've been working on the problem of separating the products.

That up above is my cell that I will be using soon. A simple U-tube with a stopper in the cathode compartment to prevent lithium from reacting with
the air. And also a stopper in the anode department with a glass tube in it to lead away the chlorine produced into a suck back bottle and then into
a base wash to decompose it. I used a similar cell for electrolysis of potassium hydroxide with good results. So hopefully this will work. The only
problem being that the lithium formed will be inaccessible while the reaction is running so I can only run it till I get a small ingot (at which time
the cell will likely draw considerably more current due to the lithium acting to expand the electrode area) then I will have to cool it down, take it
apart, and take out the lithium.
On advantage we don't have with the sodium hydroxide cell is that glass will hold up to these temps (at least borosilicate) on disadvantage is that
lithium will attack glass so that's again put out of the question. But I was wondering about high temperature silicones and such should I need a
diaphragm if I change the construction. Nonetheless, just updating those interested on my progress in this area.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Did you get any lithium formed that could be identified after the reaction or could it have been a lithium potassium alloy?
|
|
|
BromicAcid
International Hazard
    
Posts: 3244
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I could have swore I read somewhere that lithium and potassium don't form an alloy akin to the alloy formed between sodium and potassium, but that
could likely be a figment of my imagination. Anyway, I got no indication that it was lithium formed over an alloy or even over potassium. I got a
red glowing spot that occasionally caught fire and skittered around but nothing confirmatory. The crucible as you see was deep and the chlorine was
being generated all around it (though I think a lot of the chlorine formed just ate the crucible, there was smoke which could be
FeCl<sub>3</sub> . So there was no confirmatory aspect to it. . So there was no confirmatory aspect to it.
What might be useful would be a watercooled cathode. I think there is something of the like in use for calcium electrolysis where it is slowly pulled
from the surface and as it is the calcium solidifies and you end up with a rod of calcium. I didn't really investigate the solid that was left after
the melt cooled, it was very resistant to impact so I left its investigation for another day.
|
|
|
enhzflep
Hazard to Others
  
Posts: 217
Registered: 9-4-2006
Member Is Offline
Mood: No Mood
|
|
With research, photos and concise descriptions of your experiments you have many fans, BromicAcid. I for one always enjoy reading your posts, having
become familiar with the quality of analysis presented.
Was just reading 'bout your dilema and i stumbled accross this
site:
http://www.njscuba.net/artifacts/matl_metallurgy.html
Anyway, it's been too long since i did chem (10yrs ago @ school) but since Li Na and K are all in the same period(?) (column), and are all metals with
similar properties, i don't see why they couldn't form an alloy. Sure, maybe not one with definite Chem formula like with Na and K, but never the
less, seing as Al and Au can be alloyed i see absolutely no reason for this not to be possible. They may just form what the above link calls a
heterogeneous mixture of tiny crystals, as opposed to the true chemical compound that Na + K form. I suspect that all the mentions and uses of this
alloy are due to its stronger bonding, and hence better integrity under high heat conditions - as found when using as a heat transfer medium. Hope
this helps you that has helped so many.
cheers, enhzflep
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
A CELL FOR THE PREPARATION OF SMALL QUANTITIES OF ALKALI METALS
J. Chem. Ed. 31, 515 (1954)
http://www.box.net/shared/3c1ju8z6i9
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Repost of the above by request.
http://ifile.it/skohwq0
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Lithium/Al Alloy
In the electrolysis of alkali metals, it seems that one thing that
makes isolation of the metals difficult is that the metal product
is a liquid in the molten salt bath and it floats, making
containment awkward and somewhat dangerous. I've
electrolyzed small mounts of lithium before in a LiNO3/KNO3
eutectic mix at about 150°C, allowing the lithium to remain
solid. This was still difficult, because the anode products acted
aggressively against graphite and stainless steel anodes.
In the electrolysis of hydroxides, a nickel anode can be used. In
a chloride/bromide melt, a graphite anode can be used. The
advantage of these types of melts is that the anodes don't
suffer significant deterioration in normal operation, and they
are fairly attainable.
Here is a phase diagram for Al-Li. Note that the temperature is
plotted in Kelvin:

According to the diagram, aluminum melts close to 660°C, and
lithium melts close to 173°C. An alloy of the two, Li-80%/Al-
20% (atomic %) melts at about 490°C.
Below is a LiCl/KCl phase diagram plotted against degrees
Centigrade:

Between the range of 47%-77% mole % of LiCl, the salt
mixture remains molten below 490°C.
It seems conceivable that two anodes could be used: one
graphite, and the other aluminum. The anode current could be
split between the two of them, such that a solid alloy of 80%
lithium deposits at the cathode. If the cell was run at, say
450°C, then both the cathode and anodes would remain solid.
The LiCl concentration could start at 71%, and operate down
to 48% before extra salt would need to be added.
What could be done with an 80% alloy of lithium and
aluminum? It would be interesting to see how its reactivity
compares to that of pure lithium.
Anyway, here's a reference that details a process similar to
what I'm proposing. One big difference is that they deposited
lithium into an agitated molten aluminum cathode, and I'm
thinking of co-depositing the metals into a solid cathode:
Attachment: Lithium_Electrolysis_into_Molten_Aluminum.pdf (219kB)
This file has been downloaded 1039 times
Also, lithium alloy production by mixed salt electrolysis:
Attachment: 11031.pdf (1MB)
This file has been downloaded 1045 times
This reference:
Attachment: 81.pdf (286kB)
This file has been downloaded 1052 times
contains the following:
"Potassium chloride is the solvent and supporting electrolyte because it alone,
among the common alkali and alkaline-earth chlorides, has a decomposition
potential that is more extreme than that of lithium chloride. Put another way,
electrolysis of a melt comprising LiCl-NaCl will produce lithium metal containing a
substantial amount of sodium..."
So, electrolysis of a standard LiCl-KCl melt should not produce any potassium.
[Edited on 31-10-2013 by WGTR]
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Reading through http://www1.asminternational.org/asmenterprise/apd/help/Intr...
I realized that I probably misread the Al-Li phase diagram in my previous post.
If I'm not even more confused than I think, then it looks like a 70% Li alloy
remains solid up to 600K, a 60% alloy remains solid up to 800K, and a 50% alloy
up to 975K. At any of those temperatures, increasing the Li concentration causes
a molten phase to begin forming along with the solid phase.
Am I reading this correctly?
|
|
|
Motherload
Hazard to Others
  
Posts: 245
Registered: 12-8-2012
Location: Sewer
Member Is Offline
Mood: Shitty
|
|
I have been collecting Li foils from spent batteries. It isn't super pure but there still a fair amount in a spent battery.
How do I melt these foils down to get a lump ?
I can't seem to find much info on doing so.
"Chance favours the prepared mind"
"Fuck It !! We'll do it live !!"
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
I tried, it's not easy. Melting under oil at 200 C was insufficient.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Could'nt LiOH be use straightforward? (just like NaOH or KOH)?
I'm the owner of two 50 kg drums of it from a big chemical warehouse sale... 50€/drum was fine for me  (yes 100€/100kg :cool (yes 100€/100kg :cool
Just like the 1kg of AgCN for 1€, and the other chems I bought for a total of 2000€ (1€/kg or L) you don't get this often in one's chemist life.
[Edited on 26-6-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Here's my meager attempt at making lithium.

2.0g of Li2CO3 was ground together in a mortar with 4.3g of NH4NO3.

The resulting powder was heated gently in a test tube with a propane torch. Ammonia and carbon dioxide was driven off,
leaving molten lithium nitrate that gradually changed from dark brown to clear.
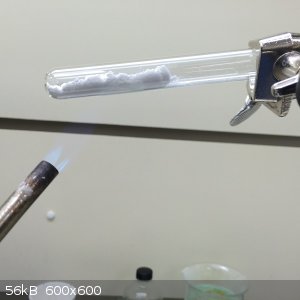
2.7g of KNO3 was added to the cooled down solid in the test tube, but the tube cracked upon reheating. For this
reason, the contents of the tube were moved to a small beaker, and reheated on a hot plate.

With the hotplate set at 300C, everything melted together as it was stirred with a stainless steel spatula.

For the anode, I choose platinum, because I can. Seriously, though, it was only $50, and it will last forever. The cathode
was just tin plated copper bus wire.

You can see the metal beginning to accumulate on the cathode wire, as well as the orange coloration from the NO2 gas.
The current was 300mA, and the voltage varied from 6-8 volts.

Here is a better picture, shown as more lithium has accumulated after about 30 minutes. It's dendritic in appearance, and
metallic looking. Keep in mind that we are using a KNO3/LiNO3 eutectic, that melts around 125C.
Lithium melts at 180C, so as long as we stay within that temperature window, the lithium remains solid in the melt. The
metal itself is protected by a thin layer of insoluble lithia, which is also a fast ion conductor for lithium ions.

Here's a short video for you that depicts the little explosions that would occur from time to time.
Attachment: burning_lithium.mp4 (1.8MB)
This file has been downloaded 979 times
Finally, here is another short clip. This one shows the reactivity of the metal with water. I noticed that it did not float, even in
spite of lithium's low density. I think this is because the metal deposit was not very dense, and was mixed with a considerable
amount of the much denser electrolyte.
Attachment: lithium_decomposition.mp4 (8.1MB)
This file has been downloaded 972 times
[Edited on 6-27-2014 by WGTR]
|
|
|
seilgu
Harmless

Posts: 13
Registered: 24-10-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WGTR  |
For the anode, I choose platinum, because I can. Seriously, though, it was only $50, and it will last forever. The cathode
was just tin plated copper bus wire.
|
That is very misleading.
I think it destroyed my platinum wire, it seems to lost its shine after being used as electrode, even after cleaning with piranha solution and flame.
Probably because lithium could form an alloy with the platinum, so it won't last forever. Lithium is known to be incompatible with platinum.
Also my quartz glassware was damaged by the burst of fire from the electrode, it formed a black spot and after cleaning I found that the surface of
the quartz beaker had melted.
Nice experiment to destroy two expensive apparatus! I want my 20 bucks back!
[Edited on 7-4-2021 by seilgu]
|
|
|
Texium
|
Thread Moved
29-11-2023 at 19:54 |
| Pages:
1
2 |