niertap
Hazard to Self
 
Posts: 76
Registered: 5-8-2011
Member Is Offline
Mood: hyper-conjucated
|
|
Experimental Chlorate synthesis
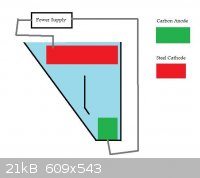
Chlorate synthesis by electrolysis:
:Solvent/ute
-Calcium Chloride electrolyte: add 100g Ca(Cl)2 per 100ml H2O
:Anode
-Carbon electrode from a battery/pencils
:all else
-stainless steel cathode-plastic container-60C-minimal pH control, possibly.
::I'm hoping the parts of the Carbon anode that are destroyed will slide back down onto the anode. This might then form the outer layer of the anode,
which hopefully takes the brunt of the corrosion. I'm thinking of trying it with some MnO2, as a much crappier anode could then be used/higher
current densities.
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
Could you post the expected reaction to take place ?
What a fine day for chemistry this is.
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
niertap do you mean for the carbon to fall down around the anode? then all the chlorate will form on top of the anode? should work shouldnt it? i mean
current will conduct through salt. i have done something similar with a nickel anode epoxied at the bottom of a shampoo bottle and filled with hcl
acid water solution. one draw back is that once the anode is gone you have to start all over with the epoxy because i doubt that current will concuct
through carbon mush. i dont think the carbon mush will harden in a solution to give you that outer layer you need but maybe it will make enough clean
chlorate on a top layer. oh! but wait,what about all the bubbling that is produced around the anode?
[Edited on 6-8-2011 by cyanureeves]
|
|
|