dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
preparation of tryptophol by baker's yeast fermentation of L-tryptophan
Tryptophol (indole-3-ethanol) was first prepared by Felix Ehrlich in 1912, who demonstrated that yeast attacks the amino acid by splitting off carbon
dioxide and replacing the amino group with hydroxyl. In a similar process in the manufacture of spirits by fermentation of sugars in grains, fruits or
vegetables, a good part of fusel oil by-product is derived from branched chain amino acids digested in analogous fashion. Ehrlich prepared tryptophol
by feeding tryptophan to a fermenting mixture and isolated the alcohol in fair yield, and noted that he also had succeeded in preparing tyrosol
(4-hydroxyphenethyl alcohol) from tyrosine through the same method.
Ehrlich's original paper is attached at the bottom of this post (it's in German). One major drawback to his original process is that he used vacuum
filtration to separate the spent yeast from the fermentation broth. Yeast sludge has a great tendency of clogging up the filter medium, and
considering the large volume involved this is a cumbersome, frustrating process.
My modification consists of using common clarification techniques used in wine making, to precipitate yeast cells and emulsifying proteins. As our
target molecule is sufficiently soluble in water (or more correctly, ~5% ethanol), the clarified solution can be simply decanted and processed
further.
Experimental
Sanitizing equipment
An 8 liter PET mineral water bottle was cleaned inside and out by washing with lukewarm water to which a drop of detergent had been added, rinsed with
tap water, followed by the addition of a solution of 1 gram potassium metabisulfite in 100 ml tap water. The bottle was shaken for 10 minutes, then
emptied and rinsed well with copious amounts of tap water then placed upside down in a drainer rack. A fitting waterlock was cleaned in the same
fashion.
Activating the yeast
In a 2 liter beaker there was placed 100 grams of (refined) white sugar and 900 ml mineral water. The beaker was placed on a magnetic stirrer and
stirred until all sugar went into solution, after which 100 grams of instant baker's yeast (Saccharomyces cerevisae) was added as a fine
stream into the vortex of the stirring mixture. The beaker was covered with aluminium foil, and left stirring until the instant yeast was fully
hydrated and a thick creamy suspension was obtained. Strong activity (CO2 evolution) was already noted within 15 minutes.
Note: It should never be left unattended for danger of overflowing and the intense foaming is best treated by stirring the thick foam
with a spatula until it breaks up.
Fermentation
In a 1 liter beaker was placed 5.37 g (0.0263 mol) L-tryptophan and 400 ml warm (55°C) mineral water, this was stirred until a clear solution was
obtained. 100 g white sugar was stirred in and when all was dissolved mineral water was added to 1 L mark, and the solution was transferred to the 8 L
PET bottle. Another 1000 ml 10% sucrose solution was freshly made and added to the bottle, followed by the yeast suspension, and finally another 2000
ml 10% sucrose solution. The waterlock was placed on the bottle, and the bottle placed in a suitable place (sink is recommended to deal with possible
overflow of foam through the waterlock). When the first 30 hours had passed another 1000 ml 10% sucrose solution was added to the broth. It was left
fermenting for a total of 5 days, after which all sugar was consumed.
Pre-clarification
To a 100 ml beaker was added 9.27 g calcium bentonite clay followed by 50 ml demineralised water. The mixture was stirred by hand for about a minute,
then left to stand for 3 hours. The supernatant liquid was decanted and the clay suspension added to the fermentation broth. After standing overnight,
the process was repeated, by letting an additional 12 g bentonite hydrate in water for 3 hours and adding the clay suspension to the fermentation
broth.
Clarification
After standing for 3 hours, 3 ml 30% colloidal silica was added. Exactly 24 hours later, 5 ml of a warm, freshly prepared 20% aqueous gelatin solution
was added. After another 24 hours standing, all suspended fine particles had settled to the bottom, leaving a clear solution. This was carefully
transferred to a broad 10 liter cooking pot using a siphon, care was taken not to disturb the bottom sediment. Subsequently, 1 liter water was added
to the fermentation flask and mixed well with the solids. After settling the supernatant liquid was carefully siphoned to the cooking pot, which now
contained about 7 liter mostly clear solution (there was a negligible amount of cloudiness which soon settled).
Work-up
Medium heat was applied so that the temperature rose to 60°C, and a fan was directed on the solution to aid in evaporation. Regular checking ensured
that the temperature never rose much above 60°C.[1] This process took 12 hours, until ~100 ml of concentrate was left. 400 ml ethanol was
added, and the precipitated solids[2] filtrated and washed twice thoroughly with 50 ml ethanol. Filtrate and extracts were pooled and the
ethanol was distilled off. To the cooled somewhat viscous concentrate was added a solution of 10 g NaOH in 200 ml water, and the mixture was heated
with stirring to 95°C for 10 minutes. After standing overnight, a brown oily emulsion with strong odor[3] was observed, which solidified
on cooling. The mixture was extracted 3x with 75 ml diethyl ether. After evaporation of the solvent, a yellow oil was left that crystallised into a
waxy solid on standing in the freezer overnight.
The solid was added to 750 ml boiling distilled water, followed by 1 g kieselguhr and a knife-tip of active carbon. After stirring for a few minutes,
the mixture was filtrated hot[4] through 2 coffee-filters, and the slightly cream-colored filtrate was reduced to about ~100 ml volume.
During cooling this turned into a white emulsion, which on standing overnight solidified into a sparkling crystalline precipitate. This was filtrated,
the crystals were air-dried for 24 hours and in a dessicator over CaCl2 overnight.
Yield : 1.95 g
The filtrate was heated and saturated with kitchen salt, which caused it to turn turbid. The cream coloured emulsion was allowed to cool, extracted
with 45 ml diethyl ether, the organic phase dried over anhydrous MgSO4, and the ether evaporated. As such an additional 0.45 g was
recovered.
Total yield : 2.40 g (57%)
melting point : 56-57°C (capillary method)
melting point reported by F. Ehrlich : 59°C [5]
melting point reported by R.W. Jackson (1929) : 58-59°C
Notes:
[1] a strong smell of yeast filled the room, adequate ventilation should be provided
[2] a substantial amount of white solids crashed out of solution
[3] resembling the odor of indole, it only became apparent after addition of base
[4] a heat gun was used to keep the suspension from cooling in the filtration funnel
[5] after an additional recrystallisation from diethyl ether:petroleum ether
Melting point (capillary method)
My melting point "apparatus" consisted of a capillary tube loaded with a tryptophol sample (crystallised from water), taped to a thermometer, immersed
into a large test-tube containing water, which was again immersed in a water-bath in which a thermocouple was placed for monitoring the bath
temperature. In such a way a fine-tuned heating of the sample could be achieved.
The melting point is a bit depressed compared to Ehrlich's but within reasonable range.
TLC (silica gel plates, detection UV 254 nm)

Solvent system used was toluene-acetone 85:15
Tryptophol (crystallised from water) was compared to indole and L-tryptophan standards, as from the indolic odor resulting after basifying the
fermentation broth concentrate, it was suspected some indole or skatole was formed as side-product. Nothing was detected in the sample however, and
the tryptophol gave a single spot.
Rf values found:
indole 0.54
tryptophol 0.13
L-tryptophan 0.00
These values were compared to the literature. Reference values found in:
"Thin-Layer Chromatography: A Laboratory Handbook", 2nd edition, E. Stahl, Springer-Verlag p 473-475 (table 88)
solvent system benzene-acetone 90:10
Rf values reported:
tryptophan 0.00
tryptophol 0.11
indole 0.53
skatole 0.56
These values are pretty much spot on, the toluene-acetone 85:15 solvent system has similar relative polarity as the benzene-acetone 90:10 from the
reference guide.
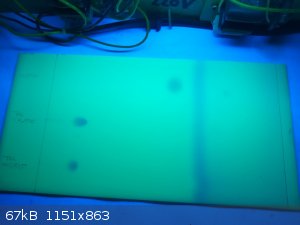
Here the crude tryptophol obtained after ether extraction of the basified concentrate, was compared to the tryptophol crystallised from water and an
indole standard. Although the crude tryptophol had a distinct odor reminiscent of cruciferous vegetables, which the tryptophol crystallised from water
lacks, the chromatogram for the crude sample shows a single spot. It is possible however that a contaminant with similar Rf value, such as
indole-3-carboxaldehyde for example, could be present.
Final remarks
Tryptophol is a plant growth regulator, it was found to be growth promoter of cucumber hypocotyl segments. It also has a hypnotic action similar to
melatonin, and may play a role in physiological sleep mechanisms. It is in fact produced by the trypanosomal parasite (Trypanosoma brucei) in
African sleeping sickness caused by the bite of the tse-tse fly.
The clarification techniques used in wine-making provide an efficient way of clearing yeast cells and suspended fine particles from fermentation
broths, making fermentation a more accessible method for the home experimenter with regards to chemical synthesis.
Attachment: tryptophol from tryptophan__ehrlich1912.pdf (363kB)
This file has been downloaded 203 times
sic transit gloria mundi
|
|
|
walruslover69
Hazard to Others
  
Posts: 236
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
That's Fantastic Work. Great Job! You were very thorough it seems with the TLC. Does this reaction of amines to alcohols using yeast work for a broad
range of substrates?
|
|
|
Texium
Administrator
       
Posts: 4665
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
Excellent work! I’d love to see a sample analyzed by NMR to confirm the identity and get a better idea of purity. There may be someone in Europe you
could send it to more easily, but if not, I’d be happy to analyze a sample for free. All I’d need would be about 10 mg in a vial, shipped in an
envelope.
|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
Gentlemen,
thank you for your kind words, it is much appreciated.
Quote: Originally posted by walruslover69  | | That's Fantastic Work. Great Job! You were very thorough it seems with the TLC. Does this reaction of amines to alcohols using yeast work for a broad
range of substrates? |
Unfortunately no, it is limited to the aromatic amino acids (Trp, Phe, Tyr), the branched chain amino acids (Leu, Ile, Val) and methionine.
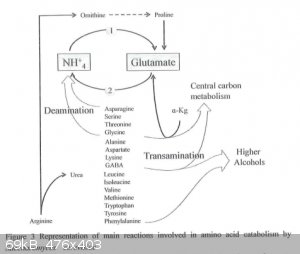 
The Erhlich pathway involves 3 enzyme-catalysed reactions: transamination, decarboxylation, reduction. In the first step the alpha-amino acid
undergoes transamination with alpha-ketoglutarate producing glutamate and the alpha-keto-acid, which then decarboxylates to form the corresponding
aldehyde, which then is reduced to the alcohol.
R-CH(NH2)-COOH --transamination--> R-C(=O)-COOH --decarboxylation--> R-CHO --reduction--> R-CH2OH
Not just baker's yeast is capable of this, but also a variety of non-Saccharomyces wild yeasts and many other microorganisms other than yeasts, such
as several species of molds and bacteria. The Ehrlich pathway is promoted by high-glucose limited-nitrogen (amino acid as only nitrogen source)
conditions. Low glucose concentration inhibits the process.
I think the process could be improved by the use of distiller's yeast (aka turbo yeast) which can tolerate up to 15-20% ethanol. Possibly allowing
more amino acid to be converted as well.
Quote: Originally posted by Texium  | | Excellent work! I’d love to see a sample analyzed by NMR to confirm the identity and get a better idea of purity. There may be someone in Europe you
could send it to more easily, but if not, I’d be happy to analyze a sample for free. All I’d need would be about 10 mg in a vial, shipped in an
envelope. |
Thanks, I will gladly accept your offer, that would be the cherry on the cake 
[Edited on 29-3-2024 by dicyanin]
sic transit gloria mundi
|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
further reading
Here's some good further reading on the process:
Ehrlich, F., Über die Bedingungen der Fuselölbildung und über ihren Zusammenhang mit dem Eiweißaufbau der Hefe.
Berichte der Dtsch. Chem. Gesellschaft 40, 1027–1047. doi:10.1002/cber.190704001156
Hazelwood et al., The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism.
Appl. Environ. Microbiol. 74, 2259–2266. doi:10.1128/AEM.02625-07
Romagnoli, G., The Ehrlich pathway for amino acid catabolism in yeasts.
Doctoral thesis, 2014
sic transit gloria mundi
|
|
|
Texium
Administrator
       
Posts: 4665
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
Tryptophol NMR Data
dicyanin sent me a sample of tryptophol for NMR analysis, and I now have the results. It looks beautiful!
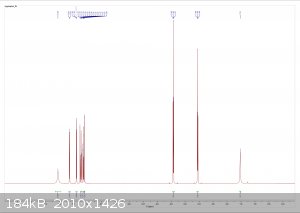 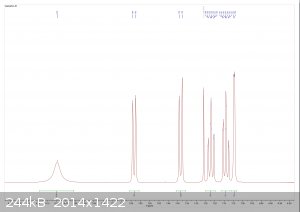 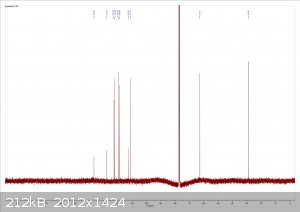
From left to right: 1H spectrum, expansion of the aromatic region in the 1H spectrum, 13C spectrum.
The spectra are consistent with the literature, and there is no significant sign of any impurities. I did notice there were some insoluble fibers
present when I dissolved the sample in chloroform (likely filter paper bits) but other than that it looks great.
|
|
|
|