bootlegin
Harmless

Posts: 1
Registered: 3-1-2018
Member Is Offline
Mood: No Mood
|
|
hho electrolysis plate configuration
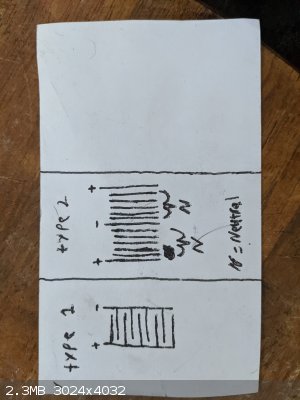
Hi! I'm currently attempting to build an electrolysis chamber for the production of hho to power one of those old 30's prest-o-lite air acetylene
torches. I've thought of two different plate configurations I could use but am stuck deciding which one. Ive included a drawing of the two
configurations I'm talking about.
Type 1 has more surface area on a single electrode and requires low volage/high current. Type 2 has less surface area and requires high voltage/low
current. Type 1 is similar to the plate configuration of a cell within a lead acid battery. Type 2 seems to be the more commonly used configuration.
I actually have another question, this one regarding plate material. Would it be feasible to reuse the plates from dead car batteries as electrolysis
plates? looking around on these forums, it seems that PbO is a good material for this and I have a ton of them in a box, most of them being in really
good condition still.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Usually type2 is used when a power supply is N.Vcell, eg 12Vdc supply
As you have many plates available you could make N.(type1) cells electrically in series.
It all depends upon desired production rate, available materials and power supply voltage and current
Don't forget to add some type of flame arrestor to prevent explosion of your cell(s)
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
How your plates should be configured is driven by the voltage of the power supply you're planning to use. Between 2-3 volts per gap is a good rule of
thumb.
Powering a flame with HHO requires obscene amounts of electricity. The highest power I've run was 1500 W and the flame produced by that was laughably
small. Very hot, but cigarette lighter sized. An ordinary medium sized propane torch is at least 5 kW, and due to the inherent inefficiency of
electrolysis you only get at most 60% flame power vs input power.
Due to the very high power, running at low voltage is impractical; you'd need massive cables, think several hundreds of mm2, and you'd lose lots of
power to resistance anyway.
From that perspective, you should use as high voltage as possible and therefore as many cells/gaps as possible.
However, as voltage increases so does the personal safety risk. Up to 80 VDC or so is fine from a safety point of view, after that it becomes
necessary to either insulate or be very cautious around the thing.
With increasing voltage comes increasing complexity as well. Sure, 10 cells is no big deal, but 100 cells may be a little unreasonable depending on
how you design and build it.
Somewhere inbetween those ends is a comfortable compromise for you, and exactly where only you can say.
I don't know how lead plates would work, but I do recommend that you use stainless steel. Using sodium hydroxide as an electrolyte, stainless steel is
almost entirely unaffected, and you don't need to consider any toxicity.
|
|
|
Parakeet
Hazard to Self
 
Posts: 74
Registered: 22-12-2022
Location: Japan
Member Is Offline
Mood: V (V)
|
|
Quote: Originally posted by Junk_Enginerd  | | I don't know how lead plates would work, but I do recommend that you use stainless steel. Using sodium hydroxide as an electrolyte, stainless steel is
almost entirely unaffected, and you don't need to consider any toxicity. |
Can I really use stainless steel as an anode? Doesn’t it dissolve and form hydroxides?
Fe —>Fe3++e-
|
|
|
Rainwater
National Hazard
   
Posts: 919
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
Just as a "what the hell" i onced cut slots into a 1in diameter pcv pipe and arranged 60 washers. Inserted that into a 1.5in pvc with caps and a vinyl
tube.
Then filled the pipe with water Hooked it directly to mains with a current meter.
Then added 2 or 3 grains of NaOH at a time until gas started being produced.
Worked like a champ until it got to hot.
Id give this one an 8/10 on the meet-yo-maker meter
"You can't do that" - challenge accepted
|
|
|
JohnnyBuckminster
Harmless

Posts: 40
Registered: 6-6-2018
Member Is Offline
|
|
I built a small cell with electrodes of steel (4 cm x 4 cm) and water/NaOH as electrolyte.
It did not work.
After a short runtime, less than 5 minutes, the electrode showed
severe signs of corrosion, and the electrolyte became brownish and warm.
I used a 6 V battery as a power source (well above the standard potential for electrolysis of water 1.3 V, so excess heat).
Replacing NaOH with KOH reduced the corrosion,
but it was still quite significant.
So I replaced the electrodes with 304 stainless steel,
which basically eleminated the problem with corrosion.
So, steel no, but stainless steel 304 yes.
[Edited on 2022-12-28 by JohnnyBuckminster]
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
I built a four plate version of type 2 using stainless steel and strong NaOH soln. Worked very well - with 20A flowing it would sustain a flame
(hydrogen/air) on the end of a 20mm tube.
I had gas separators in mine so the H2 and O2 weren't mixed until I wanted them to. And if you've seen a bag full of H2/O2 mix ignited you will know
why ( hearing protection is mandatory). Make sure you have damn good flame arrestors on your gas torch.
Best results require a large area, small spacing and reasonably concentrated electrolyte.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
JohnnyBuckminster
Harmless

Posts: 40
Registered: 6-6-2018
Member Is Offline
|
|
Quote: Originally posted by Twospoons  | I built a four plate version of type 2 using stainless steel and strong NaOH soln. Worked very well - with 20A flowing it would sustain a flame
(hydrogen/air) on the end of a 20mm tube.
I had gas separators in mine so the H2 and O2 weren't mixed until I wanted them to. And if you've seen a bag full of H2/O2 mix ignited you will know
why ( hearing protection is mandatory). Make sure you have damn good flame arrestors on your gas torch.
Best results require a large area, small spacing and reasonably concentrated electrolyte. |
My plan was to generate green H2(g) with a portable 12 V solar panel. As it turned out, PEM membranes, I purchased Nafion from FuelCellStore, are
expensive. I, therefore, scaled down everything and built a prototype using a 6V battery with two plates according to @bootlegin type 2.
I could generate some H2/O2, but it is not as easy as it might appear in some YouTube videos. When I reduced the distance between the plates, using
rubber gaskets as spacers, it got increasingly difficult to keep the gases separated, and also, I suspect there was a build-up of gas between the
plates that got stuck. So, some sort of circulation of the electrolyte is probably necessary. I didn't want to go too high in electrolyte
concentration but stay in the safe zone for the long-term survival of the membrane. Everything was quite leaky, and I never took it any further.
@Twospoons, What type of membrane did you use, how strong was the electrolyte, and how did you extract the generated H2 and O2 gases
from the individual chambers?
[Edited on 2022-12-29 by JohnnyBuckminster]
|
|
|
Admagistr
Hazard to Others
  
Posts: 363
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Quote: Originally posted by Twospoons  | I built a four plate version of type 2 using stainless steel and strong NaOH soln. Worked very well - with 20A flowing it would sustain a flame
(hydrogen/air) on the end of a 20mm tube.
I had gas separators in mine so the H2 and O2 weren't mixed until I wanted them to. And if you've seen a bag full of H2/O2 mix ignited you will know
why ( hearing protection is mandatory). Make sure you have damn good flame arrestors on your gas torch.
Best results require a large area, small spacing and reasonably concentrated electrolyte. |
Hi,Iam also building an electrolytic device for oxygen-hydrogen flame,but not HHO,oxygen and hydrogen separately.Do you have the results of your work
somewhere,so I can be inspired ?For inspiration: ?For inspiration:
https://www.aliexpress.com/item/1005003247319732.html
https://www.aliexpress.com/i/4001192601347.html
https://www.youtube.com/watch?v=gZJEDe_HUcw
https://www.youtube.com/watch?v=gH-jhN3mV60
https://www.aliexpress.com/item/1005003726628551.html
https://www.fuelcellstore.com/electrolyzer-10-e102
HHO:
https://www.enginediy.com/products/electrolysis-of-water-gen...
[Edited on 29-12-2022 by Admagistr]
[Edited on 29-12-2022 by Admagistr]
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Yes, if the electrolyte is sodium hydroxide it is almost completely unaffected. All I've noted is a very minor color shift towards darker, after
several hours of 100+ Amps.
If the anode was Iron as in you formula, then yes, it would. But it's not iron, it's stainless steel. Looking at chromium or nickel would be more
accurate.
Quote: Originally posted by Rainwater  | Just as a "what the hell" i onced cut slots into a 1in diameter pcv pipe and arranged 60 washers. Inserted that into a 1.5in pvc with caps and a vinyl
tube.
Then filled the pipe with water Hooked it directly to mains with a current meter.
Then added 2 or 3 grains of NaOH at a time until gas started being produced.
Worked like a champ until it got to hot.
Id give this one an 8/10 on the meet-yo-maker meter |
'the hell
I deeply respect your jank-level. But... No rectifier? Not much hho production with AC.
On a more serious note, cooling is really an issue on higher power cells. Mine was about 1500 W input, and efficiency is probably 50% at best. 750 W
in a mostly closed system gets hot fast.
[Edited on 29-12-2022 by Junk_Enginerd]
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Quote: Originally posted by JohnnyBuckminster  |
@Twospoons, What type of membrane did you use, how strong was the electrolyte, and how did you extract the generated H2 and O2 gases
from the individual chambers?
|
Membrane was non-woven polypropylene cloth - the type used for weed matting and cheap shopping bags. Gas extraction was via a manifold formed in the
the cell spacers, which were 6mm acrylic with cutouts as per attached image - alternating spacers were flipped left-right.
The whole stack was built up : ss plate | left spacer | membrane | right spacer| plate | left spacer | membrane etc.
There was also a small hole right at the bottom through the whole stack to allow the electrolyte level to equalize across all the cells.
Don't know the electrolyte concentration, I just tossed some NaOH in some water without measuring.
Here it is in operation
Fridays after work was always beer and science  (not always the best mix) (not always the best mix)
[Edited on 29-12-2022 by Twospoons]
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Clever and simple design. One thing that stopped me from trying a separated output cell like this was pressure. You'll be getting twice the volume of
H2 gas compared to O2 gas, right? That means the pressure between the O2 and H2 cells will naturally be unbalanced, and the O2 is at risk of being
contaminated by H2. Cloth will stop gas transfer, but not if there's any pressure behind it.
What with the VERY generous explosive limits of hydrogen concentration in oxygen, I feel like I'd never be able to trust the oxygen at least anywhere
near a flame.
Did you notice any such problems?
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
I only tried to fill balloons once. That ended badly when one balloon slipped off and the other forced electrolyte through the cell, out the other
tube, and showered the lab and me.
You can collect gas in a plastic bag though - no back pressure.
Watch the video in the link above : we were goofing off after work, tried igniting hydrogen alone (tame) and also stoichiometric H2/O2 mix (scary).
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
JohnnyBuckminster
Harmless

Posts: 40
Registered: 6-6-2018
Member Is Offline
|
|
Quote: Originally posted by Twospoons  | Quote: Originally posted by JohnnyBuckminster  |
@Twospoons, What type of membrane did you use, how strong was the electrolyte, and how did you extract the generated H2 and O2 gases
from the individual chambers?
|
Membrane was non-woven polypropylene cloth - the type used for weed matting and cheap shopping bags. Gas extraction was via a manifold formed in the
the cell spacers, which were 6mm acrylic with cutouts as per attached image - alternating spacers were flipped left-right.
The whole stack was built up : ss plate | left spacer | membrane | right spacer| plate | left spacer | membrane etc.
There was also a small hole right at the bottom through the whole stack to allow the electrolyte level to equalize across all the cells.
Don't know the electrolyte concentration, I just tossed some NaOH in some water without measuring.
Here it is in operation
Fridays after work was always beer and science  (not always the best mix) (not always the best mix)
|
Impressive! I will be back in the garage and build a new one along this idea... of course, this is the way to get the H2 and O2 out, it looks obvious
now... I did something completely different... cheers
[Edited on 2022-12-30 by JohnnyBuckminster]
|
|
|
Parakeet
Hazard to Self
 
Posts: 74
Registered: 22-12-2022
Location: Japan
Member Is Offline
Mood: V (V)
|
|
Sorry if this question is off topic.
I have a solution of sodium hydroxide, which I want to concentrate and get solid crystals. The straightforward way is to heat the solution and
evaporate the water, but sodium hydroxide solution can dissolve glass. Of course I can use a metal container, but I will have to deal with scary hot
boiling sodium hydroxide solution.
So what I thought of is, can I concentrate this solution by electrolysis? Water should become hydrogen and oxygen, while sodium hydroxide remains. Is
this a silly idea?
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Get a cheap stove (electric, gas, charcoal etc) and boil down the solution to dryness.
Do it outdoors, stay far away and/or upwind, use an iron, steel or stainless steel pan.
Definitely not aluminium.
This will give a crystaline mass of sodium hydroxide.
I've no idea how to get large crystals.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Quote: Originally posted by Parakeet  | Sorry if this question is off topic.
I have a solution of sodium hydroxide, which I want to concentrate and get solid crystals. The straightforward way is to heat the solution and
evaporate the water, but sodium hydroxide solution can dissolve glass. Of course I can use a metal container, but I will have to deal with scary hot
boiling sodium hydroxide solution.
So what I thought of is, can I concentrate this solution by electrolysis? Water should become hydrogen and oxygen, while sodium hydroxide remains. Is
this a silly idea? |
If you just want to get rid of the water, simmer it in a normal kitchen stainless steel pot. Piece of cake. That will produce a dry powder, or melt
into a difficult cake if you heat it too much.
If you want to get rid of water and don't want the scary boiling issue, set it on the stove, in a pot, at the lowest heat and point a fan at it. It'll
dry out fairly quickly, and won't spatter and misbehave.
If you want bigger crystals, just make a saturated solution and leave it alone in a jar for a while. It'll evaporate and crystallize on its own,
assuming you're not in some tropical humid place. It won't affect the glass significantly if it's at room temperature, but maybe do it with glassware
you're not super attached to, like some old mason jar, just in case it gets weakened.
|
|
|
Parakeet
Hazard to Self
 
Posts: 74
Registered: 22-12-2022
Location: Japan
Member Is Offline
Mood: V (V)
|
|
Thanks for your comments.
I now realized that electrolysis requires a lot of energy, probably more than just boiling it off. Okay, I will just heat the solution. 
|
|
|
Mateo_swe
National Hazard
   
Posts: 541
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Junk_Enginerd, you said you used at maximum 1500W and the flame produced by that was laughably small.
There surely must be a more effective way to make the splitting of the molecule, was your cell constructed without any thought of effectiveness or
optimizations done?
I have not tried this myself on any larger scale so i might be mistaken but i have seen videos of HHO cells that produce quite high volume of HHO gas.
Using 1500watts one must be able to make a decent torch at least, or is this process so inefficient?
If one is interested in HHO generators and splitting the water molecule there is one interesting source of documents and that's the supposedly
invented "Water powered sand-Buggy" built by Stanley A Meyer.
Now, i don't think for one moment that his patents and drawings can make a HHO unit that are able to power a car as he claimed, but his HHO units did
produce very much HHO gas.
If one look at the patents (i include one of those patents) and documents about it he really did a lot of work to try figure out how to split the
water molecule in the most efficient way.
Water powered car, nah, i dont think so.
But good and effective HHO generators, sure i can believe that after looking at some of his designs.
He did die under some suspicious circumstances, maybe there was a grain of thruth in his claims.
There is also a lot of good info regarding HHO generators on the net if you filter off the "Perpetual motion lunatics" and look at the more science
based info.
Maybe i should build me a HHO generator, it would be both fun and interesting.
I got most materials and a high temperature HHO torch might be good to have for something some day.
Attachment: US4936961.pdf (615kB)
This file has been downloaded 249 times
|
|
|
Twospoons
International Hazard
    
Posts: 1324
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Without appropriate catalytic electrodes water splitting is only ~50% efficient, (from memory - dont quote me!). Add in the losses in the electrolyte
resistance, especially in a sub-optimal geometry, and the efficiency could be pretty bad.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Quote: Originally posted by Mateo_swe  | Junk_Enginerd, you said you used at maximum 1500W and the flame produced by that was laughably small.
There surely must be a more effective way to make the splitting of the molecule, was your cell constructed without any thought of effectiveness or
optimizations done?
Using 1500watts one must be able to make a decent torch at least, or is this process so inefficient?
|
I can't begin to guess at the efficiency om my cell, but I don't think it was much worse than most electrolysis processes. The thing is that fire is
pretty energetic(duh). A normal $20 propane/butane torch that runs off disposable bottles is something like 3-6 kW, and they're not too impressive
really. An ordinary cigarette lighter is around 100 W.
50% efficiency is good when it comes to electrolysis. I think the theoretical maximum efficiency is about 60%.
So, to even match a normal random propane torch, we're realistically looking at minimum 6-12 kW of input power. That's approaching the limit of what a
normal household electric installation can even handle.
My setup could peak at around 2.5 kW input for shorter amounts of time, before the electrolyte started boiling off and the fuse popped. At that point
I got an angry little flame which was perhaps 4 mm across, though probably 5 cm long. Very narrow and very hot, and almost invisible
except for the sodium contamination tinting it a little orange.
Pointing it at a standard glass bottle, it would melt a 4 mm hole in a few seconds clean through. It would also deliver heat fast enough to melt
steel. Too bad you can't weld with it since both oxygen and hydrogen are the biggest enemies to steel welds lol.
It was useless for heating anything large though, since again, the total power output of it wasn't really that impressive. The contrast between the
high heat and low power was weird in that, as stated above, it would happily melt steel, but if you pointed it at an aluminium part almost nothing
happened since it conducted away the heat so much faster.
|
|
|