neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
RGA as Mass spectrometer
Mass spectrometers are incredibly useful tools for a multitude of reason,
-Checking the purity of your "homemade" stuff
-Identifying unknown chemicals
-Establishing isotopic ratio
-Determining preferred route of synthesis
-Making standards and precise mixtures
etc...
However, its not an easy (or cheap) instrument to acquire, calibrate, use and maintain.
There might be a cheaper alternative.
This is an RGA (Residual Gas Analyzer) made by MKS instrument,

It can "see" masses from 1amu to about 100amu, which is pretty good considering most volatile organics fall within this range .
It operates at fairly low vacuum and some element and chemicals can be identified easily given the proper set up:

The main chamber (lower center) is a 6 way stainless steel 2 3/4 manifold connected to the vacuum gauges (left) vacuum pumps (right) and the RGA
(top), the foreground is a low leak valve that controls the input of the chemical to be analyzed down to 10e-9 Torr.
Here is some preliminary results at about 10e-5 to 10e-6 Torr
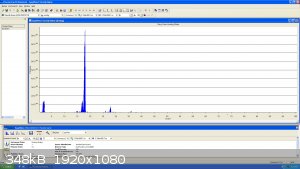
The main larger peak at mass 18 is water, which needs to be degassed for better results
This is Helium at mass 4, Monoatomic Nitrogen is also clearly visible at mass 14
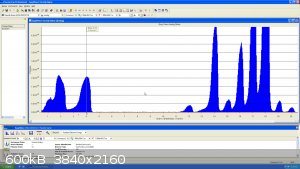
I had a little bit of Neon left from a previous experiment... here it is at mass 20, the peak looks funny because natural Neon has 2 main isotope at
mass 20 and 22...the resolution is not good enough to see the very small Ne21 (0.3%)
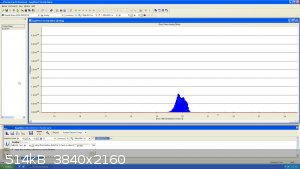
Lastly, here is a bit of DCM here with the fragmentation segment of the molecule:
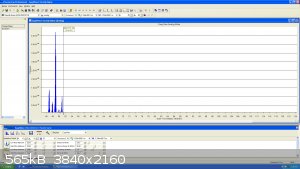
I have more stuff in store but I`ll keep it for my future video...
Would it be a stretch to ask the admin for an "analytical chemistry" forum ?
Some people are (somewhat) interested and could perhaps, benefit from sharing their experiences with lab equipment ?
Anyway, I am trying to reach higher masses and contacted MKS for the password allowing me to extend the mass spectrum. I did and it worked.. But the
quadrupole does not keep up with the software and show nothing but noise beyond about mass 98-104...If anyone here has a solution..? I am trying to
concentrate rare gasses from the air and Krypton will be in range but not Xenon (mass 136ish) ... Thanks!
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
| Quote: | | Would it be a stretch to ask the admin for an "analytical chemistry" forum ? |
This has come up before. The reality is that it is likely to have insufficient traffic to make much of a difference. Anyone looking for something in
particular is likely to be better served by a good search. Technochemistry is a good place for now.
This is an impressive piece of kit. I can see that I would have fun with one of these.
Can you give some details on how it works? How it differs from regular MS?
It would be also nice to have an indicative price -- how much would one of these things set you back?
Thus looks awesome. Thanks for sharing.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Yeah, I guess your right... I thought it would not hurt to ask, but i see how much traffic I get elsewhere and it`s obviously not a very popular
thing...
So this is a miniature quadrupole mass spectrometer that works exactly like the larger ones.
The only difference is the injection mode:
This is really the bare analytical part of the MS system and it`s up to you to feed it samples.
They were designed for ultra high vacuum residual contamination analysis, so the operator can pin point the source of contamination (grease, leak,
oil, mercury etc..)
I believe some of these devices were attached to space probes for remote analytical work of Jupiter`s atmosphere, Mars rovers, deep space solar wind
composition etc...
This one is already over 20 years old and sell on ebay for a few hundreds (150 or less is you`re lucky!)
The chamber can be just as cheap if you are patient. All the fittings, flanges and clamps usually never exceed $50 and the roughing pump can be as low
as $100 too.
The turbo pump is a different story, but again, with a lot of patience and some luck, you can get a descent turbo pump AND controller for $500..
The software is free but I had to dig around to find it :
https://github.com/HenryFBP/easyview-MKS-RGA-mass-spectromet...
There is plenty of library of mass spectrum available online for the amateur to compare spectrum of known chemicals, the main issue is to recognize
fragmentation of complex molecules and isobaric interferences.
If you see a peak at 28, it is probably di-nitrogen BUT, there is many other organics that split and fragment to yield a peak at mass 28 too...Acetone
can do the same thing with mass 44 which is commonly identified as CO2 (mass 44 too) it is entirely up to the operator to determine the likelihood of
the source sample.
Also, I wanted to use it in my fusor and look at the tritium vs helium 3 production, while both will show up at mass 3, the condition in a fusion
device (voltage, pressure, electrodes distances etc...) can affect the likelihood of either of these isotopes being present over the other.
One thing that never cease to amaze me is the 1% C13 peak that always show up next to EVERY organic peak... good times..
[Edited on 15-11-2022 by neptunium]
|
|
|
Texium
Administrator
       
Posts: 4579
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
This is a very cool idea! I always love seeing your posts on instrumental analysis. Gives me hope seeing these devices successfully used outside of a
professional lab setting. I'm a little confused on one point though: what is your ionization method, and do you have any control over the degree of
fragmentation?
Regarding an analytical subforum, I agree with J that a standalone one may not carry enough traffic, but for quite a while now I have not been a fan
of the "Technochemistry" umbrella, because it's a weird, vague term that isn't used anywhere else and contains too many disparate subjects: from
instrumental analysis, to electrochemistry, process chemistry, materials science, chemical engineering, and anything in between. I feel like we should
do something about that, but I don't know what's best.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Well Thank you Texium! I really appreciate the support !
The quadrupole has 2 filaments on the ion source at the opposite side of the detector. Yes you can control the current intensity going through it, the
alignment voltage for mass calibration, the resolution of each peak, the ion energy, accelerating voltage and electron energy. all of it is adjustable
on the software (bottom left on the last picture)
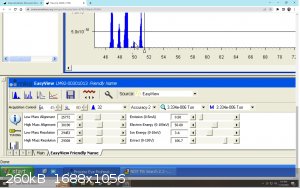
The fragmentation are more determined by the molecular bond so it`s difficult to control that. You can certainly bombard the crap out of them and
almost completely destroy it ! fun shit for sure... But this is not an inductively coupled plasma, so you can`t really "atomize" it fully...
One thing I didn`t mentioned yet but will cover in my next video...is the fragment recombining for a very brief moment and creating interferences at
different mass.. For example, Argon can attach it self to an Hydrogen atom and show up at mass 41. or a Nitrogen atom and show up at mass 54 (40+14) .
this is rare but it can happen often enough at the wrong setting.
Doubly charged species can also be a problem and must be tuned to avoid situation like Ar2+ showing up at mass 80 for example...
But don`t let that scare you, this is a fun instrument and fairly easy to get descent results ..Trust me if i did i think anybody can too..
[Edited on 17-11-2022 by neptunium]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by neptunium  | =
One thing I didn`t mentioned yet but will cover in my next video...is the fragment recombining for a very brief moment and creating interferences at
different mass.. For example, Argon can attach it self to an Hydrogen atom and show up at mass 41. or a Nitrogen atom and show up at mass 54 (40+14) .
this is rare but it can happen often enough at the wrong setting.
Doubly charged species can also be a problem and must be tuned to avoid situation like Ar2+ showing up at mass 80 for example...
|
Wouldn't a doubly ionized argon appear at mass 20? Mass 80 would be an argon dimer.
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Quote: Originally posted by Metacelsus  | Quote: Originally posted by neptunium  | =
One thing I didn`t mentioned yet but will cover in my next video...is the fragment recombining for a very brief moment and creating interferences at
different mass.. For example, Argon can attach it self to an Hydrogen atom and show up at mass 41. or a Nitrogen atom and show up at mass 54 (40+14) .
this is rare but it can happen often enough at the wrong setting.
Doubly charged species can also be a problem and must be tuned to avoid situation like Ar2+ showing up at mass 80 for example...
|
Wouldn't a doubly ionized argon appear at mass 20? Mass 80 would be an argon dimer. |
yes of course! my mistake! Thanks for pointing it out! 
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Here is a quick update,
I extended the range to 300 amu successfully. To verify everything works I introduced a small drop of mercury in the chamber.
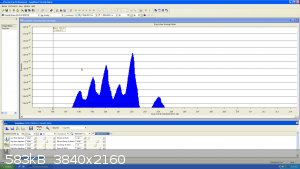
All the isotope of Mercury are clearly visible and can be matched to the known
isotopic ratio of natural mercury except Hg196 (0.1%)
So this is the scan from mass 1 to 250 AMU :
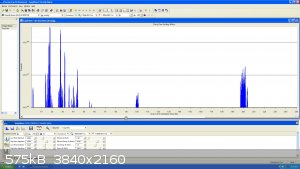
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
I was able to detect a bit of Krypton but still no Xenon, neon20 and Ne22 did show up as well... I finished the video and it was suggested to try and
detect Radon which I thought
about honestly but did not include in the video.. maybe later
https://www.youtube.com/watch?v=C3IZCxih8Rg&t=7s
|
|
|
Texium
|
Thread Moved
30-11-2023 at 10:51 |