Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
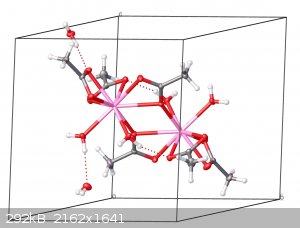
I grew crystals at home and got the structure published in the Cambridge Structural Database
It has always been my dream to have the results of amateur chemistry published on a professional level, and recently I managed to do so! Some time ago
I published the crystal structure of erbium acetate tetrahydrate in the Cambridge Structural Database.
The structure had data collected with Mo-Kα radiation at 100 K out to a resolution of 0.49 Å (!!!!) and the model managed to refine down to an R
value of 1.46%. It's a massive improvement over the previously reported structures of this compound. If you have access to the database, its
identifier is JAXMAA.
The crystallographer at UW-Madison chemistry department, Dr. Ilia Guzei, has allowed me to freely share the data - this includes the CIF and all of
the raw frames taken from the experiment. I'm not sure if there's any freely available software that will allow you to read the Bruker SFRM format,
but I might actually work on writing a converter for them myself.
The total raw data is about 5 GB and I have yet to upload the files publicly, but I will report back when I have done so.
And here are the Er acetate crystals. I grew these by dissolving Er metal in aqueous acetic acid (diluted from glacial acid) and evaporating the
resulting decanted solution. These crystals can be grown quite easily, and I am hoping to eventually grow very large crystals of them (several cm in
size) because they are stable indefinitely and do not deliquesce or desolvate.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
NICE!
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
unionised
International Hazard
    
Posts: 5135
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
:-)
|
|
|
Fulmen
International Hazard
    
Posts: 1748
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Respect!
We're not banging rocks together here. We know how to put a man back together.
|
|
|
j_sum1
Administrator
       
Posts: 6372
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Great to have you back. It has been a while.
And what you have done is quite the achievement.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2827
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
That's amazing. Congratulations! ^,^
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Congratulations, Brain&Force!
Did you not have any issues regarding supersaturation when you evaporated your Er(OAc)3 solution?
|
|
|
Liamatpm
Harmless

Posts: 48
Registered: 7-12-2016
Location: Maine
Member Is Offline
Mood: Using the process of cellular respiration to stay (barely) alive
|
|
Ok now that is pretty sweet!
|
|
|
Bmoore55
Hazard to Self
 
Posts: 85
Registered: 23-7-2018
Location: Texas
Member Is Offline
|
|
Excellent work!!! Congratulations!!!
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Quote: Originally posted by Bezaleel  | Congratulations, Brain&Force!
Did you not have any issues regarding supersaturation when you evaporated your Er(OAc)3 solution? |
Finally got back to this thread, but the acetates of Sm onward are extremely well-behaved and are currently my preferred source of lanthanide ions.
The solutions don't supersaturate to a point which causes issues. For Nd, however, the crystals are prone to decomposition (I suspect via the loss of
water) and Pr acetate doesn't form nice crystals at all.
As a side note, all the raw frame data is available by request (send me a U2U - I don't check here often but I'll get back to you ASAP). I've attached
the CIF if that's of interest.
Attachment: 2157262.cif (5.5MB)
This file has been downloaded 255 times
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
Texium
|
Thread Moved
20-11-2023 at 06:32 |
littlesky
Harmless

Posts: 22
Registered: 12-6-2023
Member Is Offline
|
|
Congratulations! Hopefully I can publish my amateur research in a professional journal as well!
|
|
|