Admagistr
Hazard to Others
  
Posts: 363
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Creating static electricity in a very vigorous chemical reactions.
Experimental generation of volcanic lightning using Popocatépetl ash and the possibility of getting a similar result in a very"stormy"chemical
process.
Years ago, I discovered a very interesting post about the creation of mini lightning bolts from imported volcanic ash in a University laboratory.
I believe that something similar could be happen in some very dynamic chemical reactions, if the design of the experiment were adjusted appropriately.
What do you think?
Does anyone have experience with creating static electricity during a very "stormy" ongoing chemical reaction?
https://lateralscience.blogspot.com/2014/08/experimental-gen...
[Edited on 30-11-2021 by Admagistr]
[Edited on 30-11-2021 by Admagistr]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
What do you even mean by a “stormy” chemical reaction? That doesn’t make any sense.
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Containing a chemical reaction to produce a jet of 100 bar pressure
to me sounds like an easy way to loose fingers, eyes, lives ec.
[Edited on 30-11-2021 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Admagistr
Hazard to Others
  
Posts: 363
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
A metal container which is electrically insulated from the ground and leaks from it CO2 under high pressure acquires a significant electrical charge.
Similarly, a hand-held foam fire extinguisher, with CO2 content, when used gains considerable electrical potential and whoever used it would suffer a
considerable electric shock if the hose of the device was not grounded. I used the word "stormy" chemical reaction in the meaning vigorous chemical
reaction that is, a chemical reaction that develops more vapor, gases, or solid particles in a short period of time and thereby increases the
pressure. An example of such a chemical reaction may be the decomposition of more amount of (NH4)2Cr2O7, the throwing of solid KOH into concentrated
hydrochloric acid...Static electricity does not have to be generated by a chemical process, but by physical processes which are caused by a chemical
reaction.
[Edited on 30-11-2021 by Admagistr]
[Edited on 30-11-2021 by Admagistr]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Seems like another complicated and inefficient way to do something simple ...As you know, metals have a tendency to loose electron while insulators
can gain electrons, hence the development of charges with enormous potential (10s or 100s of thousands of volts) but virtually NO current (except very
large discharges like lightning ). Running any fluid through an insulated nozzle will gain a net charge which is why gasoline pumps are insulated and
you should always discharge potential static before pumping gas in colder, dryer months.
Any industrial process involving the transfer of fluids with metal pipes (including the tanker truck delivering gas by the way..) is usually grounded
for that reason.
Other than that, generating large amount of gasses from a chemical reaction for the purpose of accumulating small static charges, is kind of a waste
of time, chemicals and effort in my view.
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Seeing the coulombic bang of melting sodium floating on water, I've wondered if you could put an electrode in the sodium and draw off or capture some
of the spike in voltage or in some way measure the discharge potential.
Around the 12:30 mark there're some examples of dust explosions, one demonstration using sulfur. "Using this equipment, Mr. Kent and Mr. Brown have
tested flour, rust, sulfur, coal, ...."
https://youtu.be/cUs0ix10SoY
"6. Dust storms crackled with powerful static electricity."
https://www.history.com/news/10-things-you-may-not-know-abou...
One time at night in a snowstorm in Farmington, Ct. I was in a spacious bedroom with 4 large arched windows. The thunder and lightning was tremendous.
The neat thing was the fluxuation from pitch dark to bright white with snow blanketing the ground reflecting the light.
And here some faint sparks in one example approaching 17 cm from two shower head arrangements making raindrops. I can't help but think how fun it
might be if improved with an idealised design.
https://youtu.be/698F700PM2Y
|
|
|
Admagistr
Hazard to Others
  
Posts: 363
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Quote: Originally posted by neptunium  | Seems like another complicated and inefficient way to do something simple ...As you know, metals have a tendency to loose electron while insulators
can gain electrons, hence the development of charges with enormous potential (10s or 100s of thousands of volts) but virtually NO current (except very
large discharges like lightning ). Running any fluid through an insulated nozzle will gain a net charge which is why gasoline pumps are insulated and
you should always discharge potential static before pumping gas in colder, dryer months.
Any industrial process involving the transfer of fluids with metal pipes (including the tanker truck delivering gas by the way..) is usually grounded
for that reason.
Other than that, generating large amount of gasses from a chemical reaction for the purpose of accumulating small static charges, is kind of a waste
of time, chemicals and effort in my view.
|
You see it in a very pragmatic way, as an engineer. I'm interested in a lot of paths leading to one goal, I don't evaluate whether it's worthwhile,
that it's inefficient or expensive, but it matters to me that there's another unconventional possibility...Once upon a time, our associate professor
at the University, in the subject of Atomics and Nuclear physics, when he was in a good mood, he said he'd teach us how to make gold, real gold, that
we could bring to the jeweler and he'd buy it from us and that he wouldn't be radioactive. Clearly, that would be a very complicated and expensive way
that wouldn't come off... I completely ruined his joy, when I told my colleagues that we would have to make that gold out of platinum, which is
realistically worth more.
Otherwise lightning has a very high current, even hundreds of thousands of amps or more.
|
|
|
Admagistr
Hazard to Others
  
Posts: 363
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Quote: Originally posted by Morgan  | Seeing the coulombic bang of melting sodium floating on water, I've wondered if you could put an electrode in the sodium and draw off or capture some
of the spike in voltage or in some way measure the discharge potential.
Around the 12:30 mark there're some examples of dust explosions, one demonstration using sulfur. "Using this equipment, Mr. Kent and Mr. Brown have
tested flour, rust, sulfur, coal, ...."
https://youtu.be/cUs0ix10SoY
"6. Dust storms crackled with powerful static electricity."
https://www.history.com/news/10-things-you-may-not-know-abou...
One time at night in a snowstorm in Farmington, Ct. I was in a spacious bedroom with 4 large arched windows. The thunder and lightning was tremendous.
The neat thing was the fluxuation from pitch dark to bright white with snow blanketing the ground reflecting the light.
And here some faint sparks in one example approaching 17 cm from two shower head arrangements making raindrops. I can't help but think how fun it
might be if improved with an idealised design.
https://youtu.be/698F700PM2Y |
With the Coulombic explosion question and the electric charge in the alkali metal reaction with water, it's very interesting, and yes, it
could be a case of a chemical reaction in which a significant electrical charge is released. It needs to be tested to see if any electrical
discharge can be obtained that way, as you write...Much thanks for the other videos and observations. Finally, some real answer to the point
and not a criticism that it doesn't pay off.
[Edited on 1-12-2021 by Admagistr]
|
|
|
neptunium
National Hazard
   
Posts: 989
Registered: 12-12-2011
Location: between Uranium and Plutonium
Member Is Offline
|
|
Guess I missed your point Admagistr... I was just thinking if I want large static discharge i`d get a Van de Graaff generator....And actually I did
get the exact same idea about the sodium and electrodes....I just did not think thats the kind of reaction you were talking about.
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Static electricity from jets of fluid is called the Armstrong effect and it has been know for almost 200 years.
See https://en.wikipedia.org/wiki/Armstrong_effect
"The Armstrong effect is the physical process by which static electricity is produced by the friction of a fluid. It was first discovered in 1840 when
an electrical spark resulted from water droplets being swept out by escaping steam from a boiler. The effect is named after William Armstrong,"
and here is a picture of a generator from that wiki article.
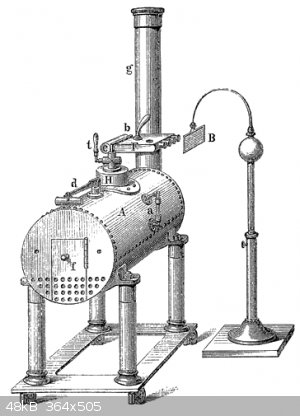
PS: Neptune: I would expect a pump (more specifically the nozzle) transferring flammable liquids to be earthed to avoid it turning in to an Armstrong
generator.
[Edited on 12/1/2021 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Admagistr
Hazard to Others
  
Posts: 363
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Quote: Originally posted by wg48temp9  | Static electricity from jets of fluid is called the Armstrong effect and it has been know for almost 200 years.
See https://en.wikipedia.org/wiki/Armstrong_effect
"The Armstrong effect is the physical process by which static electricity is produced by the friction of a fluid. It was first discovered in 1840 when
an electrical spark resulted from water droplets being swept out by escaping steam from a boiler. The effect is named after William Armstrong,"
and here is a picture of a generator from that wiki article.
PS: Neptune: I would expect a pump (more specifically the nozzle) transferring flammable liquids to be earthed to avoid it turning in to an Armstrong
generator.
[Edited on 12/1/2021 by wg48temp9] |
Armstrong's electrostatic generator was the most powerful electrostatic generator of its time, unmatched by any other. I read that no one today knows
how it was operated, how fuel was added to it without injuring the operator or possibly even killing an electrical discharge. Here's a very
interesting video. But in this video, he didn't develop its full potential because of the relatively low pressure of steam...
https://www.youtube.com/watch?v=nMuYft_EXPE&t=1s
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
I remember thinking about buying some Teflon tubing for experiments after reading this from a company that sells Teflon tubing. They warned buyers not
to use their tubing with steam. One time I bought an old fire extinguisher with the braided wire mesh over the tubing rusted and the continuity
broken. It shocked me pretty good when I went to discharge it.
The admonishment ...
"So when a liquid contacts a PTFE tube that isn’t a good conductor (white PTFE innercore), the result is phase separation and the electric charge
starts to build. The rate at which static electricity builds up now becomes a function of the fluid flow rate. When the dielectric strength of the
PTFE tube is exceeded, the electric charge will puncture the tube wall and ground itself on the stainless steel braid of the hose. Steam and fuels are
two specific areas of concern. No hoses in this catalog can or should be used in steam applications."
This electrophorus effect would be fun to try scaling up the surface area say by using four of the RMax 4ft x 8ft Thermasheath foam boards - basically
aluminum foil over Styrofoam.
https://youtu.be/axgG_aSLKPM
|
|
|
Admagistr
Hazard to Others
  
Posts: 363
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Quote: Originally posted by Morgan  | I remember thinking about buying some Teflon tubing for experiments after reading this from a company that sells Teflon tubing. They warned buyers not
to use their tubing with steam. One time I bought an old fire extinguisher with the braided wire mesh over the tubing rusted and the continuity
broken. It shocked me pretty good when I went to discharge it.
The admonishment ...
"So when a liquid contacts a PTFE tube that isn’t a good conductor (white PTFE innercore), the result is phase separation and the electric charge
starts to build. The rate at which static electricity builds up now becomes a function of the fluid flow rate. When the dielectric strength of the
PTFE tube is exceeded, the electric charge will puncture the tube wall and ground itself on the stainless steel braid of the hose. Steam and fuels are
two specific areas of concern. No hoses in this catalog can or should be used in steam applications."
This electrophorus effect would be fun to try scaling up the surface area say by using four of the RMax 4ft x 8ft Thermasheath foam boards - basically
aluminum foil over Styrofoam.
https://youtu.be/axgG_aSLKPM |
Cool, Morgan, your contributions are worth it! With the PTFE and steam, I'll try soon. Once upon a time, I made giant injections, similar to those
used by doctors, but the difference is that I made them out of one metre-long quartz tubes of optical quality, I used rubber stoppers, like a plunger,
and those stoppers were on a quartz rod. When I tried to suck the air out with this syringe, I heard a splutter, like a christmas sparkle being lit,
so I did it in the dark, and where the plunger touched the quartz glass, the place shone strongly with blue-violet light! If you think of anything
else, write ! !
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
That sounds interesting with the quartz tubes. I have several meter long Heraeus quartz tubes 19 X 25 mm that were for making fiber optic cable. I
imagine the type of rubber stopper matters and perhaps you used a lubricant to help the plunger slide or maybe friction is what works? As an aside,
rubbed with the right pressure with a damp cloth and held in the middle with a few fingers, the Heraeus quartz tubes will kill your ears with the
resulting deafening sound they make. Something like this.
https://youtu.be/sIw0Fh-fXIM
Here's this other spark making idea using pvc tubing, a paper towel, and friction.
"I have made sparks as long as seven inches (18cm). I think with a little tweaking of the configuration, they might be much longer. Incidentally,
notice the small sparks snaking around the water level at the bottom of the bottle."
Cheap High Voltage
http://amasci.com/static/foster1.html
I was able to increase the spark length from his idea by using a longer rod and keeping my distance while charging it. Also I had a teflon sheet under
my Leyden jar further insulating the system. Teflon is really good for dielectric strength.
https://youtu.be/KMQYp218a-Q
When triggering the spark by bringing the pvc rod near, it got in the way but the gap here was set at 14 inches.
https://www.pulse-jets.com/phpbb3/download/file.php?id=14228...
Even things like this watermelon or a grapefruit picked from my tree were made to produce 5 to 8 inch sparks.
https://youtu.be/0HlovqIMGkM
https://youtu.be/P-NbshIOJmc
Tidbits
"One of the largest examples of an electrophorus was built in 1777 by German scientist Georg Christoph Lichtenberg. It was 6 feet (2 m) in diameter,
with the metal plate raised and lowered using a pulley system. It could reportedly produce 15 inch (38 cm) sparks. Lichtenberg used its discharges to
create the strange treelike marks known as Lichtenberg figures." Wiki
It's interesting how some figures can continue to flicker with sparks for over an hour.
"Some specimens spontaneously self-discharge while being irradiated by the beam, causing bright flashes and bangs. The specimens emerge highly
charged. The internal layer of negative charge typically has a potential of 1.0 to 2.5 million volts ..."
https://youtu.be/9Po35g23fYI
[Edited on 2-12-2021 by Morgan]
|
|
|
wg48temp9
National Hazard
   
Posts: 784
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by Admagistr  |
Armstrong's electrostatic generator was the most powerful electrostatic generator of its time, unmatched by any other. I read that no one today knows
how it was operated, how fuel was added to it without injuring the operator or possibly even killing an electrical discharge. Here's a very
interesting video. But in this video, he didn't develop its full potential because of the relatively low pressure of steam...
https://www.youtube.com/watch?v=nMuYft_EXPE&t=1s
|
I would think operating (using) the generator would be obvious to anyone vaguely familiar with the operation of steam engines. Fill the boiler with
water then light a fire in fire box. When the boiler pressure is sufficiently high turn on the valve that lead steam to the jets preferable with an
insulated pole.
To add more fuel to the fire simple ground the system.
Check this youtube video of a small brass version of the generator.
https://www.youtube.com/watch?v=2EBmhYqW0Xk

My understanding of the charging mechanism is: charge separation occurs between the nozzle and the exiting wet steam (water vapor and droplets of
water). So its important that the nozzle is electrically conductive. I don't see how an insulating tube of PTFE or quartz could continuously provide
a connection the charge generated inside the tube.
I first read about the effect in a very old static electricity book which described a steam powered generator that could produce 6ft sparks.
Unfortunately it did not have details of the generator other than saying the charge was collected from the steam.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Admagistr
Hazard to Others
  
Posts: 363
Registered: 4-11-2021
Location: Central Europe
Member Is Offline
Mood: The dreaming alchemist
|
|
Quote: Originally posted by Morgan  | That sounds interesting with the quartz tubes. I have several meter long Heraeus quartz tubes 19 X 25 mm that were for making fiber optic cable. I
imagine the type of rubber stopper matters and perhaps you used a lubricant to help the plunger slide or maybe friction is what works? As an aside,
rubbed with the right pressure with a damp cloth and held in the middle with a few fingers, the Heraeus quartz tubes will kill your ears with the
resulting deafening sound they make. Something like this.
https://youtu.be/sIw0Fh-fXIM
No, I didn't use any lubrication at all. Friction, perhaps coupled with the difference in pressures on both ends of the rubber stopper was in play.
The rubber stoppers are Czech made and look like this, only the size was bigger. They are simply ordinary and cheap rubber stoppers. Thanks so much
for the new information ! !

[Edited on 3-12-2021 by Admagistr] |
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
I find it interesting that atomic hydrogen (aka, nascent hydrogen, also known as the H radical) is apparently formed in energetic reactions like the
action of NaOH on Al, or Zn in HCl.
So: H2 + Energy = H + H
The atomic hydrogen blowtorch is well known as a very hot flame, created by passing H2 gas through an electric arc, where the reverse reaction
apparently releases energy in the form of heat adding to the flame temperature.
Now, I surmise that static electricity is a source of energy and perhaps may similarly induce effects on hydrogen, as noted above.
Charge buildup also appears to occur particularly in suspensions. As a source of e-, one may recycle some Ferric to Ferrous or create superoxide (as
precursor to HO2 radical and H2O2) or even the one electron reduction of H2O2:
Fe+++ + e- = Fe++
O2 + e- = .O2-
H2O2 + e- -> .OH + OH-
since a Fenton reaction requires a starting amount of ferrous:
Fe++ + H2O2 -> Fe+++ + .OH + OH-
upon embedding the reaction system in a suspension, one may improve radical creation and the breakdown of unwanted organics by suggested paths above.
In effect, this is apparently noted here https://www.researchgate.net/publication/47755084_Degradatio... with a suspension of pyrite.
Also, Zinc dust likely has a significant electrostatic charge (see https://www.keyence.com/ss/products/static/resource/solution... ), where the e- presence may contribute to its noted reducing abilities (as e- +
H+ = .H, where the hydrogen atom radical is cited by Buxton as the major reducing species in acidic conditions).
[Edited on 24-12-2021 by AJKOER]
|
|
|
Mateo_swe
National Hazard
   
Posts: 541
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
If you are interested in electrostatic generation look up a Van de Graaff generator and a Wimshurst machine.
They can easily make huge voltages and they are pretty cool.
There are many types of electrostatic generator devices.
Here is a link to a page with many cool electrostatic machines.
Electrostatic Machines
|
|
|