zerodan
Harmless

Posts: 27
Registered: 4-4-2019
Member Is Offline
|
|
Making use of 12v psu for electrolysis
I have 2 server PSUs capable of putting out 70 amps @ 12V sitting at the bottom of my drawer. I'm worried of using my lab psu anymore because the
chlorine will probably shorten it's lifespan considerably, and ig I have better things to do with it.
I'm using graphite electrodes so my biggest concern so far is the anode disintegrating at such high voltage if I were to run the default
configuration.
The only other solution I considered was using some resistors/zener diodes to drop the voltage to something lower but it would have to dump a shitload
of heat into these components so they'd need to be rated for high power (thus expensive).
What I recently came up with was to try running multiple electrodes in series.
This has a few problems as per the diagram below. Assuming equal voltage drop, there would be
an approx 6V difference between each of the anodes and cathodes, I'm not aware if that would be a problem,
maybe the cathode that acts as both cathode and anode would passivate or something. I let go of the idea.
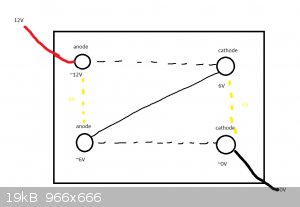
This brings us to the best one so far
What I think could work better is to have 3 completely separate cells that are to be run in series,
mitigating the issue of unintended use of cathode as anode and vice versa.
It would equate to roughly (12/3 so ~4V) potential difference which I hope would make the anodes last longer while
still utilizing the PSU to it's fullest.
Any comments appreciated 
|
|
|
unionised
International Hazard
    
Posts: 5127
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
You (pretty much) need two containers for two cells in series.
In your diagram, there's nothing (as far as I can see) to stop the electricity just running from one corner to the other and ignoring the other two
electrodes.
More cells in series is a better way to use the power from your supply. Less gets wasted as heat.
Even 6 cells (as you get in a car battery) would be workable with some reactions.
|
|
|
zerodan
Harmless

Posts: 27
Registered: 4-4-2019
Member Is Offline
|
|
Quote: Originally posted by unionised  | In your diagram, there's nothing (as far as I can see) to stop the electricity just running from one corner to the other and ignoring the other two
electrodes.
|
oh you're right  . This was a quick sketch just to show the concept and I didn't
think this through. . This was a quick sketch just to show the concept and I didn't
think this through.
Although I wouldn't completely abandon the idea of single cell in series, you could make a linear electrode array so
(12V -wire-> A1 -sol-> C1 -wire-> A2 -sol-> C2 -wire-> GND)
Here we'd have potential difference between
A1 to C1 = ~6V
C1 to A2 = ~0V
A2 to C2 = ~6V
A1 to C2 = ~12V
but the distance from A1 to C2 would be twice the distance to C1.
Resistance of solution being proportional to distance you'd maybe get same amount of current flow between A1->C1 and A1->C2.
I reconsidered this case just for fun, poke some holes in it if you can. (the obvious problem is C1 acting as an anode for C2)
Quote: Originally posted by unionised  | More cells in series is a better way to use the power from your supply. Less gets wasted as heat.
Even 6 cells (as you get in a car battery) would be workable with some reactions. |
I guess I'm making 3 cells then.
The problem is I have only single Ti cathode, and the only source available to me is china so +1 month wait time. In the mean time I want to improvise
something OTC.
I read that SS is okish as a cathode but hard to source something with high surface area.
Can galvanized steel mesh be used as a cathode? There is some in the hardware store nearby (zincate ion formation?).
What about aluminium? My first cell was 2 Al foil electrodes and sodium bicarbonate, it held up quite well but ofc in a different environment.
Never tried copper as cathode, could it work for chlorates?
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
overall 12V is too much voltage just go get a cheap meanwell PSU
|
|
|
zerodan
Harmless

Posts: 27
Registered: 4-4-2019
Member Is Offline
|
|
My definition of cheap is <12USD delivered. A quick look through ebay and I'm looking at 20-30USD + shipping, that is a lot. Recently the customs
have been acting up so I try to limit imports to what I cannot obtain for a reasonable price locally (like titanium mesh).
I picked up the 800W server PSUs for 7USD each, why not use what I already have?
[edit]
also I plan to run the cell unattended for some time. And the server psus have some crazy features and were made to be reliable, definitely wouldn't
want to damage my lab bench supply as it was quite expensive (~50USD).
[Edited on 28-11-2021 by zerodan]
|
|
|
yobbo II
National Hazard
   
Posts: 764
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Just put some cells in series. Two or three.
You can use one cell and have what is called bipolar electrodes. One side of the electrode is the cathode the other side the anode.
Will work with flat plate type electrodes. Used for plate electrodes in industry with mmo one side (the andoe) and bare titanium the other side.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
You will have some issues in this set up that will require some thought.
I don't think you mention what you intend to use your electrolysis cell for?
Not that you will get 70 A through your cell/s, but if you get anywhere near that you will need very substantial carbon anodes.
If you are running cells in series the voltage drop over the cells will vary depending on resistance within the cells, you may end up with one cell
seeing most of the voltage drop.
If you are running close to 70 A you will need serious cabling and joints, if everything is wired in series each component will see the maximum
current and will need to be treated for it.
If your power supply is not short protected you will need to wire in an appropriate fuse.
A local metal fabricator may be able to assist with your cathode/s.
|
|
|
bob800
Hazard to Others
  
Posts: 240
Registered: 28-7-2010
Member Is Offline
Mood: No Mood
|
|
See: https://woelen.homescience.net/science/chem/misc/psu.html
|
|
|
zerodan
Harmless

Posts: 27
Registered: 4-4-2019
Member Is Offline
|
|
Quote: Originally posted by B(a)P  | You will have some issues in this set up that will require some thought.
I don't think you mention what you intend to use your electrolysis cell for?
|
| Quote: | | Never tried copper as cathode, could it work for chlorates? |
Quote: Originally posted by B(a)P  | | Not that you will get 70 A through your cell/s, but if you get anywhere near that you will need very substantial carbon anodes.
|
Quote: Originally posted by B(a)P  | | If you are running close to 70 A you will need serious cabling and joints, if everything is wired in series each component will see the maximum
current and will need to be treated for it. |
It's never going to run at 70A, realistically it's going to run between 40-50A. A nice safety margin and more reliable runtime.
I'm buying 6mm gouging rods for welding, should be good enough for anodes.
Also eyed a neat SiF insulated 13awg cable for 0.6USD a meter.
I like them because they don't have the memory effect and I hope the fluoride bond is strong enough to resist any damage from stray chlorine gas.
Quote: Originally posted by B(a)P  | | If you are running cells in series the voltage drop over the cells will vary depending on resistance within the cells, you may end up with one cell
seeing most of the voltage drop. |
I know, the idea is to run 3 completely mirrored cells so they don't deviate too much in resistance. Same electrolyte, always ran together, etc.
It's a server power supply, it does way more than that. (It took me 6 months to figure out how to properly turn it on  ) )
No such thing, I'm afraid. Best I can do is a bunch of steel wholesalers but that doesn't help me one bit.
I can buy steel mesh at the hardware store (although galvanized, would be nice to know if it would be a problem or I could easily remove the zinc
only).
|
|
|
yobbo II
National Hazard
   
Posts: 764
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Down load this file and read the power supply section. There are some weird and wonderful ways to connect power to cells.
It wont matter of you put three cells in series and one 'sees' more voltage that the others. The three cells will see the same current and thats that.
Dont obcess too much about the voltage accross the cells. If the current is acceptable that all is OK.
http://pyrobin.com/files/ChloratesAndPerchlorates.zip
yOB
|
|
|
wg48temp9
National Hazard
   
Posts: 785
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
zerodan: There are youtube videos showing how these server power supplies can be modified to change the output voltage or even make it variable.
They also show what the output connections are and how to enable them.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Add one or two of these after your power supply:
https://www.amazon.com/DROK-Converter-5-3V-32V-Regulator-Tra...
I have tested it to 8A with 12V input using a resistive load.
Above 8A it supposedly needs additional cooling.
I didn't push it.
You can also make your own current limiter.
https://circuitdigest.com/electronic-circuits/voltage-contro...
Note that the article uses specific components.
I have used this design and it works for electrolytic cells where the load is basically resistive.
You can feed the opamp v+ with a variable resistor with the appropriate range and a voltage divider to give a voltage appropriate for the op amp and
shunt combo.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
I used copper cathode in perchlorate and chlorate cell but the issue with copper 2 makes the chlorate unusable for pyrotechnic purposes. for
perchlorate you wont have an issue like that.
|
|
|
zerodan
Harmless

Posts: 27
Registered: 4-4-2019
Member Is Offline
|
|
Quote: Originally posted by mysteriusbhoice  |
I used copper cathode in perchlorate and chlorate cell but the issue with copper 2 makes the chlorate unusable for pyrotechnic purposes. for
perchlorate you wont have an issue like that. |
You mean Copper +2 ions? Why would they be a problem?
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
copper 2 ions in chlorate compositions can lead to room temp sudden ignition due to instability of copper chlorate.
|
|
|
zerodan
Harmless

Posts: 27
Registered: 4-4-2019
Member Is Offline
|
|
I messed up  . .
I wanted to remove the copper coating from gouging rods by running them in a sacrificial batch so Cu -> Cu(OH)2 but apparently some of it plated
out on my Ti cathode (should've used a copper plate, I just wasn't expecting the Cu to stay in solution).
Would using a slightly copper plated cathode produce significant quantities of Cu2+ to be dangerous?
Can I add NaOH and filter to get rid of Cu ions?
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
copper coated Ti is a blessing in disguise if you seen the PbO2 thread.
ofc it would have to be a proper plating on the Ti and not just shit.
|
|
|
CRUSTY
Hazard to Others
  
Posts: 139
Registered: 5-6-2016
Location: Nearby
Member Is Offline
Mood: High-Order
|
|
Personally I use a PSU from an old desktop PC and although it can't put out 70A, it'll happily do 30A at 13V and also has 20A outputs at 5.0V and
3.3V. Having those extra outputs greatly simplifies getting the voltage right. I'd highly recommend looking for one from a PC to anyone else looking
to try this, it's so much easier than stepping down the 12V output and they're very easy/cheap to find used.
|
|
|
Rainwater
National Hazard
   
Posts: 925
Registered: 22-12-2021
Member Is Offline
Mood: Loving every second
|
|
Make and model of the psu in question please?.
Most server sized power supply have a fixed current limiter, with slight modifications to the hardware or software settings, this can be converted
into a current source.
"You can't do that" - challenge accepted
|
|
|
KCLcoal
Harmless

Posts: 7
Registered: 30-8-2019
Member Is Offline
|
|
I have run a chlorate cell with a 12V PC power supply. Connect 3 cells in series.
It should be 12V/3=4V per one, but actually there was a big difference. About 5V, 4V and 3V respectively. In addition, the amount of current flowing
was small, and fluctuations over time were large. So using 12V is not wise,
As some have already pointed out, you should use a 5V power supply. The voltage drop will be about 4.5V, and you should be able to get a moderate
voltage.
|
|
|
woelen
Super Administrator
        
Posts: 8016
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
With a 12V power supply I would go either for three cells in series, but this is a little too much. You only have 4 volts per cell, and theoretically
it can work, but if your cells are somewhat smaller and you have a little more series resistance in your cell, then one flaky cell may spoil it for
all cells, due to the series connection.
Another option could be using two cells in series, but then add a few big power diodes (even diodes for 10 A are cheap). You could start with three
diodes in series, but if the current is too low, just remove one, if the current is too high add one. Each diode takes 0.6 to 0.7 volts. Good cooling
of the diodes may be necessary.
If you want to perform electrolysis at tens of amperes, then the diode trick may become problematic, but still, one can get diodes with much higher
max current, but these can be pricey. In that case, you could go for power resistors or even long runs of wire, which introduce a few hundreths of
ohms of resistance. Do not make a coil of the wires, keep them free, so that they can easily get rid of their heat.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
I run chlorate cells at around 3.7 volts anyway at 200ma/cm^2 with electrode distance of 2cm apart with 2 cathodes 1 anode.
if you want low operating voltage do the following:
1. copper plate or use bolts to secure the connections to the electrodes.
2. pH control lowers the operating potential by a lot run at 60C or higher with Ca/Mg chloride buffer for pH control to be viable and not a Cl2 gas
generator.
3. run saturated NaCl
4. electrode spacing 2.5cm or lower
5. 2 cathodes for 1 anode, 3 cathodes for 2 anodes ...
|
|
|