symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
What chemistry are you working on.
Just curious what other are working on for projects or discussion of future projects
Mine are:
Hydrazine sulfate
Nitric acid
Moldable thermite
LiH
SOFC fuel cell
high test hydrogen peroxide
Potassium peroxide (CaC2 + KF) (O2)
Sodium pernitrite
Copper peroxide
Zinc peroxide
(Other peroxide salts)
Plastic to gasoline like mixture
Pine to gasoline like/turpintine
FFC Cambridge electrolysis of calcium chloride to isolate titanium
For the fourm would be
The 2021 newsletter
What about everyone else??
[Edited on 30-4-2021 by symboom]
|
|
|
IrishJeremy
Harmless

Posts: 11
Registered: 12-11-2019
Member Is Offline
|
|
A perchlorate cell, chloroform syth, waiting on KNO3 to arrive for nitric acid, aluminum powder for testing tetraamine copper nitrate NPEDs, and a
chlorate cell.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I have some amides I'm planning to make, so the students can have them as unknowns in September. I also want to play with some FC alkylations and
Williamson syntheses, just to become a better organic chemist.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Some of my projects have been:
- Purifying KNO3 from otc low level fertilizer (done)
- Purifying and extracting pretty much anything otc by all means. Displacement reactions have been one of my most intriguing area, as there is nothing
more satisfying than mixing two bulk compounds and crashing out or crystallizing desired (or undesired) stuff from a solution, and extracting the
wanted compound.
- Nitrite synthesis from it (done, although the product was mix of K and Na)
- Benzaldehyde synthesis, using nitrite catalyst (done)
- Lead dioxide anode with sulfuric acid electrolysis from cast 20x10x5mm lead sheet (tba)
- And further, a working chlorate cell - I also attempted chlor-alkali, but it ended up an eternity project as well
- Dehydrating ethanol (MgSO4 + CaSO4 were unsuccessful, CaO failed because I ran out of gas and haven't ordered sieves yet)
- Hydrazine hydrate from urea hypochlorite sulfate method, and then distilling from base
- Research on per-compounds (persulfate, percarbonate, perborate) and concept of extracting H2O2 from percarb with urea adduct or direct pyrolysis to
distill H2O2 from the adduct)
- Piperine extraction (2kg of black pepper yielded 50g of recrystallized pure, sad)
- Synthesis of ammonia (urea+naoh), HCl (Na bisulfate and salt, or HCl and CaCl2)
- Hexamine (tba)
- Phenylacetaldehyde (got my lab all stinky, but never got it in any meaningful quantities, the isomerization with silica gel turned out not so well)
- Benzene (have thrice been already preparing it, but never got to actually doing it as I did have no need for it and got better things to do)
But, most importantly, I've been training my lab skills and techniques and gotten quite a bit of experience doing all kinds of experiments, my goal is
to actually do some complex synthesis instead of just pushing stuff around.
My hoarding personality does not quite fit with flying all around through the whole scale of chemistry. I get excited about something, I may look what
I need for it, and see that 5kg bucket costs only a little more than 1kg, so I end up with one. Just in case, you know. And it doesn't neither help
that, over 10 years ago stuff was much less available than it is now. All the online stores, all the otc stuff with required MSDS, and then the great
vendors on this forum that can get you anything you need.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Right now the only chemistry I'm working on is in the research lab, but here's a summary of the reactions I'll be running in there. So far I've only
done the first Suzuki coupling. Classes and teaching/grading have mostly kept me out of the lab this semester, but soon enough I'll have a lot more
time!
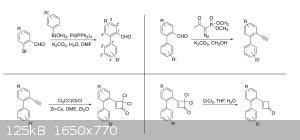 
|
|
|
paulll
Hazard to Others
  
Posts: 112
Registered: 1-5-2018
Member Is Offline
Mood: It's fine. Really.
|
|
+1 for hydrazine sulphate
...after the sulphuric acid drain cleaner distillation that I'm working up to now the temperature outside is no longer amenable to liquifying Chlorine
More immediately:
-Benzoic acid from toluene
-More salicylic acid from aspirin, much of which is intended to become phenol
-Finally turn that pile of eggshells into Calcium acetate
Longer-term, I'm thinking DDT.
And at least 1000 other things, of course.
What is that you're working on, Texium? Interesting-looking molecules, there.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Trying to see if I can make a
new type of cyclization work (the second screenshot) which would be a relatively easy way to access an interesting 6-7-6 tricyclic structure that
shows up in a number of natural products. But in order to get to that part, I need to make the biarylcyclobutene precursor, which takes four steps.
|
|
|
ChemTalk
Hazard to Self
 
Posts: 65
Registered: 13-12-2018
Location: United States
Member Is Offline
Mood: colloidal
|
|
We are working on a series of compounds of rare-earth elements, we just finished with neodymium, and made ten different compounds. Video going up
soon!
|
|
|
Belowzero
Hazard to Others
  
Posts: 173
Registered: 6-5-2020
Location: Member Is Offline
Member Is Offline
|
|
My 2 main projects are:
Birkeland eyde/arc process
Contact process
After building a whole bunch of prototypes I finally have a working and stable birkeland setup, I certainly underestimated the difficulty of building
such a thing.
I've been reading just about every book related to the subject and tried to incorporate those elements.
Also it is interesting to learn where a lot of setups I've seen went wrong.
Since my lab is not in my house I am stilly not fully confident to leave it running unattended for long periods of time.
As a side project I am working on making a decent Cognac from white wine.
Some interesting recent discoveries such as aging with ultrasonic and the use of UV light are worth looking into.
|
|
|
Xanax
Hazard to Self
 
Posts: 54
Registered: 28-8-2003
Location: Sweden
Member Is Offline
Mood: Curious
|
|
I'm mixing my precriptions to be more effective, for example; Doasage for clonazepam (in swedish): http://richardhandl.com/files/Kemi/Laboration%202%20-%20Flyt...
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
IIRC BaO2 barium peroxide is possible to make by keeping hot BaO above concentrated H2O2 (80%+)
H2O2 can be concentrated down by fractionally boiling off water, with quite little H2O2 loss, plante experimented on this and i believe he had maybe
20% by weight loss of H2O2
im working on platinum electrodes
|
|
|
charley1957
Hazard to Others
  
Posts: 159
Registered: 18-2-2012
Location: Texas
Member Is Offline
Mood: Roasting
|
|
Yesterday I made a couple of batches of ethyl acetate for my bug killing jar. Lately I took apart some large wet cell Nicad batteries to try and
extract cadmium. Not much luck there. Suggestions welcome. Besides that just cleaned up several kilos of very dirty Mercury, 5cc’s at a time. Glad
that’s done. It took days. Right now doing some mods to the lab. Adding a on/off switch at the bench for my water supply pump, and replumbing the
water supply to the bench and fume hood. I decided the off switch was too far away when I developed a big water leak during a distillation the other
day.
You can’t claim you drank all day if you didn’t start early in the morning.
|
|
|
Bedlasky
International Hazard
    
Posts: 1239
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Why would you do that in this way? If you heat BaO at 500 °C, you get peroxide. Or you can precipitate BaO2.8H2O from alkaline H2O2 solution.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Trying to ethylate salicaldehyde with ethyl bromide to give ethoxybenzaldehyde. Using essentially the same conditions as for ethylating naphthol, but
it's just not working. Bah!
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
I had a chumk of lithium exposed to air for an extended period. It became a muxture that included a considerable amount of nitride with some oxide and
in all likelihood a decent amount of hydroxide and carbonate. I am converting to carbonate of hopefully reasonable purity. It is drying right now.
|
|
|
ViktorVaughn
Harmless

Posts: 3
Registered: 30-11-2021
Member Is Offline
|
|
Hi, this is my first post here. I'm a chemical engineering student but I'm just getting started with amateur chemistry, as opposed to working in a
university lab. I wanted to start by getting very basic equipment and doing something simple. I decided to extract melatonin from tablets. Why? Mostly
because that's something I had laying around and it seemed simple enough, but I also had the idea of maybe someday making 5-methoxytryptamine and then
serotonin from it.
The process was very simple. I simply crushed the pills, added some ethanol as solvent, heated it a little bit (I don't think this was necessary but I
did it anyway) and filtered off all the stuff that wouldn't dissolve. All the other components of the pill had very poor solubility in ethanol so I
figured I didn't need a separatory funnel or anything for this. I let the ethanol evaporate and when it dried this off-white powder was left.
Now, I haven't further purified, characterized or even weighed it because I still lack some very basic equipment. I might try to see if the melting
point checks out. I also might do TLC on it when I get around to buying some silica plates.
I wanna gradually advance to more interesting projects as I acquire more equipment, solvents/reagents and so on. Next I'm thinking of purifying
acetylsalicylic acid from aspirin and hydrolyzing it to salicylic acid. Synthesizing methyl salicylate ("wintergreen oil") from the salicylic acid
would be cool but I'm not sure how doable that would be for me in the near future.
(As I said, it's my first time here so I hope the content and placement of this post is appropriate.)

[Edited on 2-12-2021 by ViktorVaughn]
"Viktor used the college—used its modern scientific equipment to conduct strange, forbidden experiments."
|
|
|
Bedlasky
International Hazard
    
Posts: 1239
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
I plan to do some refractometry measurments. And then maybe try some bromatometric titrations (specifically determination of hydrazines and phenols).
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Collecting and ampouling Nitrogen dioxide. Converting periodic acid of uncertain composition to pure metaperiodate and pure orthoperiodate.
If you're not part of the solution, you're part of the precipitate.
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Converting a separation funnel to a pressure equalizing addition funnel
Putting together bits of hoses and doing some very amateurish glass blowing from some glass drinking straws to convert a separation funnel to a
pressure equalizing addition funnel so I can build a Cl2 generator.
The Cl2 generator I'm planning to use for making some sulfur dichloride (SCl2) once I work up the energy and feel safe enough to
attempt it.

[Edited on 3-12-2021 by Monoamine]
|
|
|
Bedlasky
International Hazard
    
Posts: 1239
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by nezza  | Collecting and ampouling Nitrogen dioxide. Converting periodic acid of uncertain composition to pure metaperiodate and pure orthoperiodate.
|
What periodates exactly? I guess KIO4 and Na2H3IO6? Have you some plans for them?
|
|
|