maqce
Harmless

Posts: 11
Registered: 12-11-2019
Location: Bordeaux, France
Member Is Offline
|
|
Calcium chloride recrystallization
Hi everyone,
In few days I will receive 2kg of CaCl2 from a druggist. It's not super pure and since id like to use it for drying solvants, I planned to
recrystallise it, but I don't know what solvant to use : CaCl2 is very soluble in alcohol and water.
Do you know any solvant that could work ? Not to hard to find if possible
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Can't you distill after drying? I don't see your route going anywhere.
|
|
|
maqce
Harmless

Posts: 11
Registered: 12-11-2019
Location: Bordeaux, France
Member Is Offline
|
|
The problem isn't the solvant to dry, it's that i don't know which solvent to use in order to recrystallise the calcium chloride, to purify it before
using it as a drying agent... but I don't know in what I will recrystallise it since it's very soluble in both alcohol and water
[Edited on 29-3-2020 by maqce]
[Edited on 29-3-2020 by maqce]
[Edited on 29-3-2020 by maqce]
|
|
|
Sulaiman
International Hazard
    
Posts: 3723
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
if it is just contamination by old solvents that concerns you,
would it be sufficient to heat the CaCl2 until molten, allow to cool and then pulverise it ?
This will also ensure that the CaCl2 is anhydrous.
It may be sufficient to just heat to above 260oC to get anhydrous CaCl2 as most solvents will have evaporated by that time.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
maqce
Harmless

Posts: 11
Registered: 12-11-2019
Location: Bordeaux, France
Member Is Offline
|
|
I'd like to purify it because it's not made for lab initialy, I bought it from a common druggist... For instance, on their website they specify that
there is about 3% of NaCl.
Here are the specs :
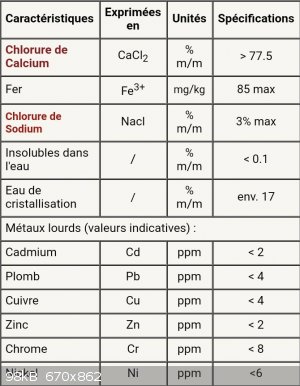
(Sorry its in french) "eau de cristallisation" is just the water from the hydratation
[Edited on 29-3-2020 by maqce]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I meant; dry your solvent with the CaCl2 as it is now, filter and then distill your solvent to get rid of of the impurities. I don't think you can
easily recrystalize CaCl2.
Also, NaCl probably won't hurt anything you want to do with your solvent.
|
|
|
Morue
Harmless

Posts: 11
Registered: 3-4-2020
Location: Canada
Member Is Offline
|
|
If your main impurity is NaCl, I think you could use ethanol to remove most of it. NaCl is poorly soluble in ethanol.
Solubility in ethanol:
(75°C) NaCl : 0.077 g/100g (Journal of Chemical and Engineering Data, Vol. 50, No. 1, 2005)
(70°C) CaCl2 : 56.2 g/100g (Wikipedia)
Dissolve in EtOH, filter impurities and boil off all the solvent to recover your CaCl2. Anhydrous EtOH is hard to get though, might be worth trying
with isopropanol.
|
|
|