vanBassum
Hazard to Self
 
Posts: 66
Registered: 16-4-2019
Member Is Offline
|
|
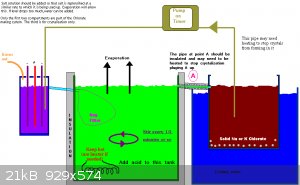
KClO3 cell continuous production
I have a chlorate cell witch is working like it should. But there is one drawback to this setup. The potassium clorate witch forms at the bottom of
the cell gets stuck from time to time. The reason, I think, this happens is because of the lower temperature at the bottom of the cell. So I opted to
improve this process.
The idea is to slowly pump the electrolyte to a separate container with a filter in the bottom. Because of the low flow, the temperature in the
separate container will be held at ambient temperatures. Therefore the potassium chlorate will precipitate out of solution en be caught in the filter.
The filtrate will find its way back to the cell. This way the product can be easily collected from the filter while the process isn't disturbed. Only
thing left to do other than collecting product will be to add saturated KCl to the cell whenever needed. (Witch could be automated.) There is one
potential problem. The potassium chlorate could crystallize out inside the tubing witch will clog the system. This can be fixed by heating the tubing,
but i don't know how practical this will be.
For now I created a small setup like this to test the theory. I've added a peristaltic pump to pump the electrolyte around. The filter consists of an
empty soda bottle slightly raised above the cell. Any filtrate can flow back trough a tube to the cell. How well the soda bottle will keep up... Time
will tell. It is easily replaced with something else if necessary.
This is running now for a little while now. Ones I have results Ill post them. 
BTW, I can imagine that a setup like this is not very practical for most hobbyists because I don't know what to do with all this chlorate. It is
however quite interesting to mess about with setups like this.
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
Here is a solution, use pure NaCl as your electrolyte and have a separate tank hooked up via pump where the temperature of the tank is lower and KCl
solution is dripped onto the flowing NaClO3 to convert it to KClO3 which would build up at the bottom of the tank and using NaCl electrolyte is
overall better for your anode
|
|
|
mysteriusbhoice
Hazard to Others
  
Posts: 477
Registered: 27-1-2016
Member Is Offline
Mood: Became chemistry catboy Vtuber Nyaa
|
|
If the second tank has agitation then they won't build up in the walls and can easily be filtered but I would say that this kind of setup is overkill
for small scale
|
|
|
vanBassum
Hazard to Self
 
Posts: 66
Registered: 16-4-2019
Member Is Offline
|
|
So I tried this...
The idea kind of works but I had a lot of issues with crystals growing in the tubes.
I tried to adjust the speed of the pump, but my pump is not fast enough.
For now the conclusion is that this is more trouble than its worth. I you want a lot of CLO3 just make a bigger cell.
Although this was a bit of a failure, I might try this again in the future.
A much faster pump every ones in a while could suck up the crystals from the bottom of the cell, dumping them in a filter and returning the liquid
back into the cell.
|
|
|
Deathunter88
National Hazard
   
Posts: 519
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
KClO3 just isn't soluble enough for a continuous cell to work well. As mysteriusbhoice said, use NaCl and then react with KCl afterwards.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Its a good idea in principle, and not only for the reasons you mention.
Commercial operations in fact alos use large holding tanks, and the main reason is that it allows time for a slow but efficient hypochlorite
autooxidation reaction to take place.
The holding tank is kept hot though (IIRC around 70 deg C) to speed up the reaction.
Thanks for posting your attempt, I've thought about trying this many times, but never got around to.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
yobbo II
National Hazard
   
Posts: 763
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Some have done it.
The solubility is not a problem. If using sodium chlorate you will have to wait a while before chlorate starts to come out as solid due to its high
solubility.
Yob
Attachment: carl_tauch.pdf (1.2MB)
This file has been downloaded 553 times
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
i would advice you to have 2 solutions of electrolyte
also, if you use MMO to add a bit of sodium chromate
the 2 solutions so that you can swap solutions and keep it going like that
the 2 solutions being sodium chloride/chlorate
ontop of that you just pour in an excess of sodium chloride, as the sodium chloride is spent it forms sodium chlorate, this crystallizes out into big
nice cubes of sodium chlorate, you will never quite get around a bit of digging, but you just pour out the warm saturated NaClO3 solution and let it
cool down, once the NaClO3 is done crystallizing out (give it maybe 24H) you can use it again, dousing the whole deal with NaCl
anyhow while this crystallization is taking place you just flip in the other solution
NaClO3 seems so much more easy for chlorate production, ontop of that, whatever you fancy using KClO3 for, you can also use NaClO3 for, it has even
more uses than KClO3 and is a stronger oxidizer with the drawback of being a bit hygroscopic
its also much easier to purify as it has much higher solubility than KClO3, finally converting your already once crystallized NaClO3 should give you a
more pure product than your first crystal crop of KClO3
to avoid chlorine gas, and maybe even catalyze the whole reaction you may add a small amount of NaOH to the electrolyte, this will help absorbing
chlorine gas faster
the times ive had a cell running, i just used a plastic lid with no gas vent, and magically the chlorine gas was never an issue while taking the lid
off inside, the plastic lid also kept tight as the water condense was forming at the top, very crude and almost too good to be true setup.
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
add water to chlorate cell witch. she should melt right away.
"A mind is a terrible thing to lose"-Meisner
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
Quote: Originally posted by Antiswat  | i would advice you to have 2 solutions of electrolyte
also, if you use MMO to add a bit of sodium chromate
the 2 solutions so that you can swap solutions and keep it going like that
. |
Hexavalent chromium is toxic .i use nicrome wire as the cathode.
"A mind is a terrible thing to lose"-Meisner
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
sodium chromate is not hexavalent chromium, despite that electrolysis electrolytes are rarely recommended for consumption anyhow. for sodium chromate
to turn hexavalent you need to acidify it, and if you do that with chlorate solution you have a much bigger problem under your bed.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Sodium chromate definitely is hexavalent chromium! CHromate (yellow) and dichromate (orange), both are hexavalent, they can be converted into each
other by adding acid or base, but that does not change the oxidation state of the chromium!
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
then ill have to fall back on that and argue that you only use very small quantities, and that sodium chromate also tastes a lot better than the
dichromate- anyways!
|
|
|
yobbo II
National Hazard
   
Posts: 763
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
"i would advice you to have 2 solutions of electrolyte
also, if you use MMO to add a bit of sodium chromate"
There is no need to add chromate or dichromate. It only helps a small amount (stops hypo-chloride being converted back to chloride at the cathode,
also called cathodic reduction). There are many other things you can do to get the current efficiency high.
The chromate addition is not linked to anode type (whether or not you have MMO or graphite etc etc) its a 'cathode thing'. Use a small
cathode and that will do the job OK (lower cathodic reduction).
Woelen did a great demonstration of a chlorate cell and cathodic reduction.
https://woelen.homescience.net/science/chem/exps/miniature_c...
If added you will have to remove it from the final product. Barium chloride can be used as barium chromate is very insoluble.
This means you have to obtain barium chloride etc etc. You may have some Ba in the final product etc etc. Better to have a green cell.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If you make potassium chlorate, then it is remarkably easy to remove the chromate/dichromate. Potassium chlorate can be recrystallized very well and
after just two recrystallizations no chromate can be observed at all. The chlorate has a very good tendency to purify itself (at least from the
chromate). Even the first batch, directly from the cell, only has a very pale yellow color. Given the intense color of chromate/dichromate, even at
extreme dilutions like 1 : 10000, this means that the problem of removing chromate is not a real problem with potassium chlorate.
If you make sodium chlorate, then things are more difficult. It is a lot harder to make pure sodium chlorate, because that does not crystallize that
nicely and it is quite hygroscopic. At the high concentration, needed to crystallize this, also quite some part of the chromate is included in the
solid. So, I would say, go for KClO3, and use a little chromate/dichromate.
|
|
|
vanBassum
Hazard to Self
 
Posts: 66
Registered: 16-4-2019
Member Is Offline
|
|
True, but for sodium its more of a pain.
I get around this problem by reusing the filtrate to top of the cell. When the cell has run for a while I collect the electrolyte, boil it down and
set the solution to cool. The crystals are collected and further processed. The filtrate, which is now way less than what I took out of the cell is
dumped into a bucket containing 'electrolyte' consisting of chlorate, chloride and chromate. When its time to top off the cell, I just use whats in
that bucket. This way I don't really loose my chlorate that is otherwise lost in the purification.
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Chemical formula
CrO2−4
and Cr2O2−7
Chromate is an ion that contains one chromium atom (in its +6 oxidation state) and four oxide atoms. Its formula is CrO4. Its overall charge is -2.
(Wikipedia)
Hexa- is the Greek prefix for the number six, from hex, "six";
[Edited on 3-24-2020 by CharlieA]
|
|
|
yobbo II
National Hazard
   
Posts: 763
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Antiswat  |
NaClO3 seems so much more easy for chlorate production, ontop of that, whatever you fancy using KClO3 for, you can also use NaClO3 for, it has even
more uses than KClO3 and is a stronger oxidizer with the drawback of being a bit hygroscopic
. |
Why The WiZ gets pains in his round-and-fuzzies when someone
mentions-- SODIUM CHLORATE
A message on the West Coast Pyro Board and a recent article in
"Special F/X Newsletter" have made mention of the use of Sodium
Chlorate. There are one hundred and two really good reasons why
this oxidizer has not found use.
Reasons number; one, two, three -> # one hundred--IT IS NOT SAFE MIXED WITH -- ANY THING. PERIOD.
WANA DIE YOUNG? USE SODIUM CHLORATE!!!
See this thread
https://www.sciencemadness.org/whisper/viewthread.php?tid=28...
Yob
|
|
|
yobbo II
National Hazard
   
Posts: 763
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Going back to this and looking on line the enthalpy of formation of Sodium chlorate is about -391 and not -53 as stated in the referenced thread.
It's about the same as k chlorate.
I guess there are other reasons?
Yob
|
|
|