Jango
Harmless

Posts: 7
Registered: 23-11-2010
Location: Oxfordshire, England
Member Is Offline
Mood: No Mood
|
|
Does more current increase the rate of electrolysis?
Hi,
I'm interested in doing various experiments, including electrolysis of various solutions. I'm searching for DC power supplies, and have found some
good ones on eBay. I want the electrolysis to be done as fast as possible (i.e: larger amounts of chemical to be formed at each electrode per second).
Should I look for a DC power supply that has a higher voltage and a lower current (e.g: 20V 2A)? Or should I look for one with a lower voltage and a
higher current (e.g: 13.8V 15A)?
I've been looking for something that has a constant, reliable supply so that I can leave it running so that the electrolysis finishes relatively
quickly (also why I am only electrolysing small quantities).
I can't afford the ones that have a massive voltage and current, and I only want to electrolyse relatively small amounts at once (e.g: 100-200ml of a
1M solution).
Thanks. 
[Edited on 23-11-2010 by Jango]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The only thing which counts is current. By means of electrolysis you perform redox reactions at the electrodes. At the cathode you 'push' electrons on
the reactants and at the anode you take electrons from the reactants.
Current is a measure of charge per second, which is a measure of number of electrons per second. At a current of 1 A, appr. 1/96500 mole of electrons
are pushed on the reactants at the cathode and the same number of electrons is taken at the anode.
As you can see, at a current of 1 A you need to 96500 seconds to have one mole of electrons transferred to/from reactants. If you want to make e.g.
chlorate from chloride, then you need 6 electrons for each transformation of chloride to chlorate, so making 1 mol of chlorate requires 6*96500
seconds of electrolysis at 1 A. This is almost one week!
So, you want high current.
The voltage determines how much current is going through your cell. Part of the voltage is required for doing the redox reactions, the remaining part
is just consumed as heat. Typical redox potentials are in the order of magnitude of 2 volts for both reactions at anode and cathode summed together.
You also need to take into a few tenths of volts for overpotential at the electrodes. Altogether this makes up a minimum of around 3 volts before any
reaction occurs.
If you want a decent current, then you also need some voltage for ohmic resistance. If this resistance is e.g. 2 ohm, your overpotential is 1 volt and
the total redox potential is 2.5 volts, then you need a voltage of 5.5 volts if you want 1 A of current:
5.5 volts - 2.5 volts redox potential - 1 volt overpotential --> 2 volts remaining
these 2 volts remaining give a current of 1 A if the ohmic resistance is 2 ohm
Summarizing: Take a voltage of 5 volts for many electrolysis experiments (a simple PC powersupply does the job). If you want more versatility, take a
voltage of 12 V, combined with a resistor network.
I have written several pages on the subject of electrolysis and making a decent very cheap power supply. The first link tells how to make a good and
versatile power supply, suitable for a lot of electrolysis experiments and even suitable for small to medium scale production work if you also keep
the 5V. The second link is an example of the use of this power supply and the making of a chemical by means of electrolysis. The third is a more
theoretic page, which gives insight in the electrical characteristics of an electrolysis cell.
http://woelen.homescience.net/science/chem/misc/psu.html
http://woelen.homescience.net/science/chem/exps/miniature_ch...
http://woelen.homescience.net/science/chem/exps/electrolysis...
[Edited on 23-11-10 by woelen]
|
|
|
Jango
Harmless

Posts: 7
Registered: 23-11-2010
Location: Oxfordshire, England
Member Is Offline
Mood: No Mood
|
|
Thank you for your good reply. 
I've tried to convert an ATX PSU into a DC power supply before, but it broke. I haven't really got the equipment to build one properly, and I would
expect that buying a 15A bench supply would be better, as it would be more reliable.
So, if more current is important, then I will go with the 15A one instead of the 2A.
Is 15A enough to, for example, fully electrolyse 200ml of a 1M solution of NaCl in 12-24 hours? Or would I need more than that for it to be more
successful?
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Jango  | | Is 15A enough to, for example, fully electrolyse 200ml of a 1M solution of NaCl in 12-24 hours? Or would I need more than that for it to be more
successful? |
At this point, you might consider asking for help with how to do the computation rather than
asking for someone to do it for you. Here's my contribution: look up the Faraday constant.
|
|
|
Jango
Harmless

Posts: 7
Registered: 23-11-2010
Location: Oxfordshire, England
Member Is Offline
Mood: No Mood
|
|
So, according to Faraday, if I was to do the electrolysis in one second, I would need 96,500A.
But I want to do it in 12 hours (43,200 seconds).
So, the minimum current I would need = 96,500/43200 = 2.234A am I right?
Or is it more complicated than that?
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Not much more complicated than that, but your units are off. Yes, I
know you put down amperes and seconds as units, but there are more units lurking (and it's not hours). Read up on dimensional analysis and you'll
figure out what you're missing.
|
|
|
Jango
Harmless

Posts: 7
Registered: 23-11-2010
Location: Oxfordshire, England
Member Is Offline
Mood: No Mood
|
|
Sorry, I can't work out what I'm missing.
I thought:
*96,500 coulombs of charge needed per mole of electrons. So, using the I = Q/t equation, if I wanted 1 mole of electrons to be electrolysed in 43,200
seconds (12 hours), I would need a current of: I = 96,500/43,200 = 2.234A.*
I also thought (but I think I'm wrong):
*1 mole of electrons = 6.02 X 10^23 electrons. In 1 molecule of NaCl, there are 28 electrons (10 in Na+, 18 in Cl-). Therefore, I would need 28 times
the amount of charge (and thus, 28 times the current) to electrolyse 1 mole of NaCl in a certain period of time. So 2.234 X 28 = 62.552A. I hope I'm
wrong. A power supply with that much current would be above my budget!
But looking at Dimensional analysis (on Wikipedia), I didn't see anything about electrolysis on there. Please could you possibly give me a bit more
guidance? I don't mean to be lazy, but I'm only a 15-year-old British GCSE student interested in a bit of electroysis, and all of this is above my
level (apart from the I = Q/t equation, I learnt that at school).
Thank you for all of your help so far. 
[Edited on 24-11-2010 by Jango]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
"mole" is a unit you didn't use in your previous
posting. Dimensional analysis is not electrolysis-specific, it's about making sure all your units are accounted for. The Faraday constant has units,
your current has units, etc., and you'll need to always ensure that you don't have any left over. | Quote: | | In 1 molecule of NaCl, there are 28 electrons (10 in Na+, 18 in Cl-). |
In this context, 28 is wrong, as are
both 10 and 18. It's time to go read up on "half reactions".
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
One ampere-hour equals 3,600 coulombs, thus 26,8 amp-hours equals one mole of electrons; or your 1st answer of 2.34 amps for 12 hours.
The number of electrons involved is just the ionisation ones, Na(1+) Cl(1-), not the total electrons in each atom - it takes a lot more energy to pull
of more than one electron from a sodium atom.
|
|
|
Jango
Harmless

Posts: 7
Registered: 23-11-2010
Location: Oxfordshire, England
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by not_important  | | One ampere-hour equals 3,600 coulombs, thus 26,8 amp-hours equals one mole of electrons; or your 1st answer of 2.34 amps for 12 hours.
|
So, was I right originally?
Quote: Originally posted by not_important  | | The number of electrons involved is just the ionisation ones, Na(1+) Cl(1-), not the total electrons in each atom - it takes a lot more energy to pull
of more than one electron from a sodium atom. |
Oh yes, you're right. I forgot that! As if sodium would lose ALL of its electrons! I obviously wasn't thinking. 
[Edited on 25-11-2010 by Jango]
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
If you want to force a lot of current through your cell, you will have to decrease its electrical resistance. One thing you could do is set up
multiple cells in parallel. What this will do is decrease the combined resistance.
The general idea.
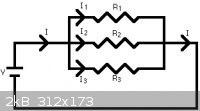
The more cells you add, the lower the total resistance, the more power you will draw, and the more amps will go through your cells.
|
|
|
bdbstone
Harmless

Posts: 18
Registered: 23-4-2011
Member Is Offline
Mood: No Mood
|
|
One way to have high current power supply is to use welding machine.. Max.current goes up to 150A.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
It might be capable to run through a current of dozens of amps, but remember:
V=IR
As resistance goes up, current goes down, no matter how powerful your welding machine might be.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
More current = faster reaction, but there is are limits. Pushing 95000 amps through a 100 ml cell for one second probably won't work, depending on the
reaction you want to try. Why not?
Well (if we ignore the magnetic field that will make your cell explode) some of the intermediate reactions may proceed at a comparatively slow rate.
For instance, for making chlorate from chloride, it is necessary for chlorine evolving at the anode to dissolve for it to participate in a subsequent
reaction taking place in the bulk of the solution (not at the electrode). The solubility of chlorine is limited and increasing the chlorine evolution
(by increasing the current) beyond the rate at which it is consumed by the reaction will only result in losing chlorine gas to the atmosphere.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Obviously there are limits, I'm just saying that putting several small cells in parallel will allow you to draw more power and more amps than if you
set up a giant tub in line with the power source. One more question remains, why does the OP want to speed up an electrochemical reaction? I would
rather use less power and leave the reaction overnight rather than pump hundreds of amps and crossing my fingers that nothing blows up.
Also, one more thing that the OP should consider is that passing more amps will generate tremendous amounts of heat. This can cause thermal runaway,
cracking glass etc. etc...
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
White yeti, my previous post was not in response to you but I disagree. There is no difference between using two cells in parallel or using a single
big cell with twice the volume and electrode surface area. Not in terms of resistance, yield, etc. anyway, provided the distance between the
electrodes remains the same when scaling up the cell. This is easy to see if you imagine a cell and then imagine extending the length of the
electrodes by a factor 2. The result is: half the resistance, as it would if you had two cells in parallel of half the size.
The only real difference I see is that a big cell will have a smaller surface to volume ratio, making passive cooling less efficient. Big cells will
run in to cooling problems sooner.
There is a clear advantage to multiple cells if you have a fixed voltage power supply that is high enough to feed several cells in -series-. Or if
there is a long distnce between the power supply and the celll, so you need to use a higher voltage to minimise losses in the wires runnging to the
cell. Then it helps to run cells in -series- from the higher voltage.
Otherwise,you can in principle simply increase the voltage to increase the rate of the reaction in a single big cell. The rate is proportional to the
current. The price you pay is reduced power efficiency (more heat).
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Phlogiston, you were probably asleep in physics, but take a look at this formula:
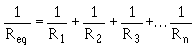
If you plug in multiple resistances, it turns out that the overall resistance is smaller than your lowest resistance (if arranged in parallel). Going
back to good old V=IR, if voltage remains constant, current increases when resistance decreases.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
I did meet my wife during physics classes so I may not have been paying attention as much as I should 
However, electronics being another hobby of mine, I am very familiar with that formula.
Let me introduce another formula, the resistance of a wire:
R=rho*L/A
rho = specific resistance of whatever the wire is made of
L=length
A=cross sectional area
Think of a cell as a wire. For simplicity, imagine a cell consisting of a 1x1x1 cube, two opposing sides of which are the electrodes. Then A=1, L=1,
and (let's assume rho=1) therefore R=1. Now take a cell of 2x1x1 instead of 1x1x1, with correspondingly larger electrode. Then A=2, so R=1*L/2 = 0.5.
As I said: increase electrodes surface area by 2, and maintain the same distance over the surface area and the resistance will be half.
Putting two cubes in parallel will have exactly the same effect, with the same total electrolyte volume and electrode surface area so you gain nothing
by constructing two cells:
Req = 1/(1/1 + 1/1) = 0.5
Same result.
For more complex cell configurations, consider that current can flow from every point on one electrode to every point on the other electrode, via
every possible path through the cell. Integrate over all these paths to find the resistance. I assure you the result will be the same, however.
[Edited on 16-9-2011 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
This is valid for the bulk resistance of the electrolyte, but is not a full
electrical model of a cell, which has to include electrode polarization, amongst other things. For the point you were making, though, it's OK. The
right target is specific electrode current, which you can't improve by parallel cells.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
What you are saying is correct and I like your analogy.
However, if you want to get very technical, you are missing some key factors. The fact that the electrodes are larger does not necessarily mean that
current is evenly distributed throughout the electrode. Going to your example of a rectangular prism, 2x1x1, it depends where you place the wires if
you want to use all the electrode surface area available to you. You could find wires that have a cross-sectional area as large as your electrode
(good luck).
Practically,when your electrodes get big enough, another factor comes into play. When you connect an electrode to your power supply with a wire, (the
wire has negligible resistance, unless it's very thin), the cross section is no longer the entire surface area of the electrode. If you think of your
electrode as a playing card, your cross sectional area is about the area of the shortest edge of the card, pretty darn small, (if not smaller due to
uneven current distribution). Considering the fact that the cross sectional area of your electrode does not increase much when you increase the total
electrode surface area, resistance stays about the same, whether your cell is big or small. The only way to increase the cross sectional area is to
make your electrode thicker, (a big waste of electrode material). In a practical situation, I still think connecting up several small cells is both
more economical, safer and capable of handling higher currents, than a giant cell.
[Edited on 9-17-2011 by White Yeti]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Many excellent points have previously been pointed out, yet I do want to underline how many variables complicate this topic.
I have followed this thread with a degree of interest as I have enjoyed per / chlorate electrolysis cell. experimentation for a few years now. From
what I (and a friend) have determined is that the amount of variables that INTERACT is somewhat profound. So a simple query such as amount of current
in relationship to yield is complicated by solution density, pH (& pH management) spacing of the electrodes, temperature of solution, type of
anode/cathode construction, & depth / size of electrode contact area within the electrolytic solution. - That's a great many variables!
I was lucky enough to have teamed up with an experienced friend and we were able to cut some good deals on both MMO/Ti and graphite electrodes. I had
been using computer power supplies with success yet was able to find some professional (fan cooled) switching power supplies, one of which is 150amps
(@5vdc). There has been some material written which demands construction further from the simplistic designs. Pumping cells, current pulsing, auto pH
handlers and even more professional designs that take considerable work to start up. Yields and crystal formation have been fantastic. yet to
categorize this higher yield(s) from one element alone is very difficult. pH level itself can alter the efficiency of current usage toward final
output yield.
Thus far if one were to have a switching PS such as a computer PSU and to monitor pH carefully I believe those to be the most consistent contributing
factors - yet such a thing as solution temp and timing of pH adjustment can easily allow a 30a unit to preform as well as a non-pH adjusted higher
current unit. I have had difficulty with containerization beyond 5 gallons due to cost and design issues. However I have found methods of reduction of
graphite material and degradation through pH. I encourage people to "branch out" toward varying techniques - but records and notes become extremely
important as the variable compound upon one another.
I encourage the use of a digital pH meter. Even an inexpensive one is perhaps one of the more valuable tools I have used. I also found that array and
spacing require a great deal of trial and error and solution volume and density should always be recorded in conjunction with this type of experiment.
Slight alterations can (at minimum) cause extra work and possibly confuse weeks of well written notes.
Personally I found that larger cells have an advantage in that they can produce visual yields quicker and make the endeavor rewarding in that source
current may be utilized with a wider spread.
Chromates for stabilization have also become a worthwhile addition. I also believe that it may be a useful shortcut to purification processes.
[Edited on 20-9-2011 by quicksilver]
|
|
|