avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
heating media's for heat bath ?!
there are chemical process's which i got to reach high temperature as 290c, and apply them on my vacuum system.... so i cant use direct heat source(i
could make thermal cracking on my glass vessels).
i was looking for a readily available chemical to melt in my steel pot and allow me transfer the heat to my system, i already try calcium chloride
mono hydrate, this chemical loose his lest water molecule under 290 Celsius and become solid.
i was looking in wikipedia for month to find a good salt and cheap which allow me to do this, and i field !!!
so if someone know a material which melts in 200->350c +- ,easy to get, and friendly to steel and glass, please write it here.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Mixed alkali metal nitrates and nitrites are the old standards, as are sand or small metal (steel) shot, and low melting point alloys. A mix of 53%
KNO3, 7% NaNO3, and 40% LiNO3 is used over the range of 150 to 450 C. Silicon oils can be used up to ~300 C, but are expensive.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
Mg(NO3)2, KNO3, NaNO3 mixture is good too...not sure of exact proportions
oh...KNO3, NaNO2 is old standby for metal heat treating...
[Edited on 6-19-2010 by Eclectic]
|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
i don't have access to lithium .... the 150 to 450 range is perfect, someone know similar mixture which allot me to work in those temperatures ?!?!
the mixture don't corrode my steel pot?
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I would be scared of dropping anythign organic in that molten nitrate/ite bath! :O
How about wax? you can buy high m.p/b.p. wax for such purposes.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Waxes generally don't go above 250 C, most are limited to below 200 C.
Nitrate baths are used because they have reasonable low melting points and are compatible with many ferrous alloys. Alkali hydroxides have lower
melting points, but absorb water and CO2 from the air, and needless to say are hard on skin and many fabrics. Chlorides either have too high a
melting point, or react with moisture; chlorides and ferrous metals don't get along well especially if they are exposed to air.
From Vogel:
| Quote: | | A satisfactory bath suitable for temperatures up to about 250° may be prepared by mixing four parts by weight of 85 per cent, ortho-phosphoric acid
and one part by weight of meta-phosphoric acid; the mixed components should first be heated slowly to 260° and held at this temperature until
evolution of steam and vapours has ceased. This bath is liquid at room temperatures. For temperatures up to 340°, a mixture of two parts of 85 per
cent, ortho-phosphoric acid and one part of meta-phosphoric acid may be used : this is solid (or very viscous) at about 20°. |
Ordinary phosphoric acid can be used if you first heat it with stirring to about 20 C higher than the intended temperature of use, it partially
dehydrates to polyphosphoric acids.
Molten metal baths are another choice, Wood's metal being the classic but because of its cadmium content it presents fume toxicity problems.
50/50 In/Sn 118-125 C
58/42 Bi/Sn 138 C
In 157 C
63/37 Sn/Pb 183 C
Sn 231.8 C
Pb 327.5 C
Metal baths generally are bothersome to work with, with some alloys the oxide film can reach with the glassware, slowly damaging it over many uses.
Salt baths are much easier to clean up after, because of their water solubility. Often glassware was coated with soot or graphite before being placed
in a metal bath, this lower the amount of metal and oxides sticking to the glass. Both metal and salt baths need to be melted before the flask can be
placed in them, and when done you'd best remove the flask while the bath is still liquid.
There's a handy discussion at http://www.ilpi.com/inorganic/glassware/heatsources.html including 2 salt mixes - one K/Na NO3 and the other those plus NaNO2
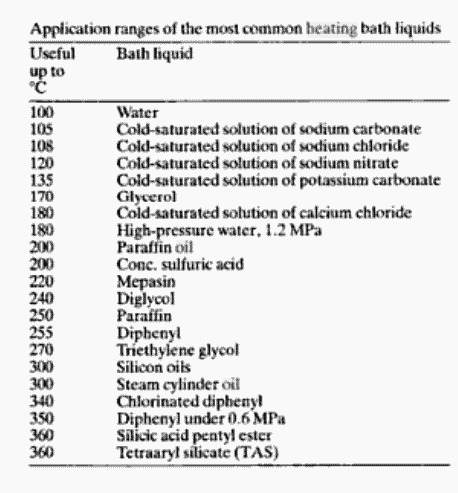
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by not_important  | | Molten metal baths are another choice, Wood's metal being the classic but because of its cadmium content it presents fume toxicity problems.
|
RotoMetals has a selection of low melting point alloys, many of which don't have cadmium (although most of the lower-melting ones do). In addition to cadmium, they're various
alloys of bismuth, lead, tin, and indium, and in one case, antimony.
As a small digression, the antimony bearing one is an equivalent to Indalloy 217-440. The numbers are temperature in Fahrenheit. It's a non-eutectic,
and these numbers are the solidus and liquidus temperatures. I mention this as interesting because that temperature range seems remarkably large. It's
evidently used for fixtures in machines, as it expands almost half a percent a day after solidifying. So don't let it harden around your glass.
|
|
|
CouchHatter
Hazard to Others
  
Posts: 152
Registered: 28-10-2017
Location: Oklahoma
Member Is Offline
Mood: 76 elements taken!
|
|
Could someone explain why the "useful up to __°C" metric is roughly half of the compounds' boiling points? Diphenyl can be used right up to its bp.
I have lots of glycerol and wonder about using it at slightly higher temperatures. I know it won't blow up or anything, but I'm curious how they
arrived at the specific temperatures.
|
|
|
wg48temp9
National Hazard
   
Posts: 783
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
I would use a material that in the event that the flask fails its contents does not react violently with the material. For example organics and
molten nitrates are probably very bad. As almost 300C and vacuum is beyond the recommended operating conditions for a borosilicate flask its got a
higher chance of failing.
Consider using molten tin.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
The eutectic mixture of biphenyl and diphenyl ether (aka Dowtherm A) is apparently a useful heat transfer medium usable at high temps.
Attachment: preprints201806.0048.v1.pdf (1.1MB)
This file has been downloaded 795 times
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Heating mantles are usable to 450oC
You could also wind-your-own nichrome or kanthal heating coil directly on the pot (with e.g. fibreglass insulation layers)
Also, AFAIK hot phosphoric acid is not compatible with borosilicate glass,
and may also corrode steel (despite the phosphate passivation layer.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
what you need is "sand" bath with instead of sand try using sand sized aluminium grains. you can look for them in local metal working shops they have
Metal swarf from from machining.they even give them for free.because of Al much higher thermal conductivity so much better than sand or a liquid that
can spill allover .i use this set up to make con.H2SO4 .
"A mind is a terrible thing to lose"-Meisner
|
|
|
yobbo II
National Hazard
   
Posts: 762
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
Could brake fluid be used?
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
At a pinch, you can use cooking oil.
https://en.wikipedia.org/wiki/Smoke_point
or engine oil
https://en.wikipedia.org/wiki/Motor_oil
[Edited on 10-4-20 by unionised]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by CouchHatter  | Could someone explain why the "useful up to __°C" metric is roughly half of the compounds' boiling points? Diphenyl can be used right up to its bp.
I have lots of glycerol and wonder about using it at slightly higher temperatures. I know it won't blow up or anything, but I'm curious how they
arrived at the specific temperatures. |
Short answer: Vogel 5th ed. starting on page 71; or give it a try, you'll see.
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
I was wondering of using nuts and shims that are sold at hardware store for like 5 bucks a kg. Find the smallest one, like 4mm and so on, and get a kg
or two and use them as steel heating bath?
Actually.. Could one use these to form a sort of heating mantle pit?
Or would it be a potential for catastrophy to go so far as to cast half-sphere from aluminum that is about the size of a flask?
EDIT: Oh, if anyone wouldn't have thought it already. How about this?
https://www.amazon.com/Chemglass-CG-1992-54-Anodized-Aluminu...
[Edited on 15-5-2020 by Refinery]
|
|
|
Fulmen
International Hazard
    
Posts: 1716
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
According to simple physics the most important factor should be bulk thermal conductivity. Low conductivity would cause more delay and thermal losses,
but it won't stop heat from flowing.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
I recently formed lead shots by pouring molten lead through a sieve.
Could aluminum be formed the same way? Small Al shots would form a great heating bath.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
You can use Wood's alloy or various metal low-melting alloys, but it's more as a possibility, as it is quite expensive.
|
|
|
Mateo_swe
National Hazard
   
Posts: 541
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Wouldnt buying a heating mantle be easier and less work?
You could buy these cheap replacement heating mantle sleeves and use them if you have a powersupply or a variac to control the power.
I have ordered some of those and plan to control them with a PID Temperature Controller, a solid state relay and a thermocouple.
Only thing is they dont have stirring, but that is quite easily made with a fanmotor and a magnet.
Is there any applications where a bath would be preferred over a heating mantle (except with low temps and sensitive materials as for example
distilling ether)?
|
|
|
Texium
|
Thread Moved
29-11-2023 at 13:28 |