aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
'Continuous' distillation?
So with a potentially long and aggravating task of distilling 25L of a 23% ethanol:water mix soon (from fermentation), I'm wondering what the best
method for distilling all of it would be without buying a large still dedicated for this one task.
I've come up with the following procedure and I'm looking for a bit of feedback (I searched the forums and haven't come across anything):
Bucket with 25L 23% ethanol:water mix, siphon hose leads to a inverted funnel placed over a Buchner funnel loaded with diatomaceous earth and
activated carbon (and sealed). Buchner funnel downspout connected to tubing which leads into one neck of a 3 neck flask.
The 3 neck flask (500mL) is fitted with:
Neck 1: the tubing from the filter (via a thermometer adapter with a short glass tube)
Neck 2: a Vigareux column (to subsequent distillation apparatus),
Neck 3: a second tubing adapter (connected to neck #3, improvised from a thermometer adapter and a glass tube which reaches down to the bottom of the
flask).
The second adapter connected to tubing leads to a vacuum flask via a stopper in the top, with the side arm to vacuum.
Loading the still
To load the still, the glass tube on neck #3 is withdrawn carefully to avoid pulling the solution out, and vacuum is applied. The ethanol is drawn
from the bucket, through the filter, and into the flask.
Unloading the still
Once the ethanol portion has been distilled and the vapor temperature begins to increase, the glass tube is inserted, the adapter on neck #3 is
removed, and vacuum is applied to pull out the water remaining, and the flask is reloaded (the water is pulled into the vacuum flask and disposed of
before reloading).
This could potentially save me from having to cool down the flask/oil bath, carefully add the ethanol:water mix via funnel and wait for the Vigreux
column to heat up again.
Now the one snag that I could forsee is that normally you would dispose of the first small fraction which might contain methanol, however in this case
I would end up distilling both the ethanol and methanol to concentrate, and then redistill a second time to yield 95% ethanol free of methanol.
So what are peoples thoughts on this procedure? I'm not necessarily producing the ethanol for consumption, moreso towards either soxhlet extractions
(eg: capsaicin), or reactions to form ethyl esters (thus requiring a methanol-free distillate).
|
|
|
densest
Hazard to Others
  
Posts: 359
Registered: 1-10-2005
Location: in the lehr
Member Is Offline
Mood: slowly warming to strain point
|
|
Have you seen drawings of countercurrent continuous stills? Very simply, it's a vertical column with side outlets over a heated pot with an outlet.
The feedstock is continuously run in from the top and is heated by rising vapor. Once the system stabilizes, there is a temperature gradient hottest
at the bottom and coolest at the top of the column. At the point where the desired product condenses, the vapor or condensate (depending on design) is
taken off. The waste is continuously removed from the pot.
Here's a rough sketch of something one might put together out of reasonably standard glassware. The input rate would need to be controlled along with
the heat to keep the takeoff point just above the boiling point of ethanol. Wrapping the whole assembly with some form of insulation is probably
necessary to keep the gradient constant: aluminum foil as a heat reflector, glass wool/foil would be better. The input flows down the side away from
the takeoff point.
A second, higher takeoff might be necessary to condense and discard the more volatile fractions so they don't accumulate in the column or leave at the
top making an explosion hazard or poison the operator. 
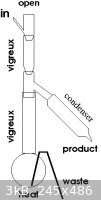
[Edited on 23-11-2009 by densest]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
As a chemical engineer, I would say that a scaled-up continuous distillation apparatus is what would be used for industrial-scale separations of
liquids, on a much larger scale. This would certainly be the case for commercial production of gin and whiskey (with up to about 50% ethanol) for
drinking, and, with a much longer column, approximately 90%-ethanol "grain alcohol". An apparatus like that above made from laboratory glassware would
be called a "pilot plant".
|
|
|
stateofhack
Hazard to Others
  
Posts: 123
Registered: 6-5-2008
Location: Warm Coast
Member Is Offline
Mood: annoyed
|
|
hahahahaha..harsh
I have set-up a to distill 5 L of 32 % ethanol (home-made) a while back. I just did 2 L batches, bigger i can't do 
I reccomend tho having some activated charcoal in your vigreux column (lightly packed), it seems to come over much clearier then!
Best of luck!
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
I've just about finished distilling some ethanol from a similar feedstock (16 liters of ~17% alcohol fermented stuff). I used a 2 liter RBF w/ 24/40
neck and a Hempel column packed with little sections of 6mm tubing. Still had to do many runs obviously; the column is nice (gets the alcohol content
over 90% from 16% on first run through and then to 95+% on the second run) but I end up only putting 1400g of the fermented stuff in the flask each
time around.
Anyway, I notice from the smell that my product is still contaminated. It smells slightly sweet, foul, and fishy. Googling a bit I see that
alkylamines are a likely impurity; given the boiling points and such I suspect some of the contaminants might be n-butylamine, butyraldehyde, or
similar. Can anyone recommend a good way to remove these impurities? I could try another distillation with activated carbon in my column, but if there
is another way I'd like to hear about it.
|
|
|
Satan
Hazard to Others
  
Posts: 126
Registered: 1-5-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by bbartlog  |
Anyway, I notice from the smell that my product is still contaminated. It smells slightly sweet, foul, and fishy. Googling a bit I see that
alkylamines are a likely impurity; given the boiling points and such I suspect some of the contaminants might be n-butylamine, butyraldehyde, or
similar. Can anyone recommend a good way to remove these impurities? I could try another distillation with activated carbon in my column, but if there
is another way I'd like to hear about it. |
Its better to let it stand with carbon for one day, shaking it few times, then filter it and distill.
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
Let your product sit for a day or two and it will smell like ethanol. Whatever that fishy smell is, it seems to be very volatile and evaporates off
even in a sealed bottle at least in my personal experience.
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
I have the same issue with my home brew alcohol.
It has this horrible smell and taste, not that it is so strong but it smells old, I don't know any better description.
Having tried a lot of things such as leaving it over activated carbon for a week with regular shaking.
Re distilling it twice (that makes 3 times :S ) throwing away the below 78 deg C and stopping before the temp rises etc.
I am quite desperate at this point.
I have even been thinking chemical means of cleaning it up but that would make consumption a more doubtable issue.
So i have 3 litres of dirty 95% EtOH 
What a fine day for chemistry this is.
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
User: pitch in a tablespoon or so per litre of anhydrous sodium carbonate. It helps by gently hydrolyzing all the esters that form. Also it helps to
pull out some water from the ethanol at higher concentrations. I think you could at least get it up past the azeotrope by just using sodium carbonate
to absorb water. I'm sure if you are worried about trace amounts of sodium ions, you could redistill.
I haven't drank mine yet, just for lab use really, so I can't tell if it works or not for the taste.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Let your product sit for a day or two and it will smell like ethanol. Whatever that fishy smell is, it seems to be very volatile and evaporates off
even in a sealed bottle at least in my personal experience. |
If it were actually volatile (more so than EtOH), distillation should drive it off (though I can certainly believe that there are or were additional
impurities like methylamine that would be eliminated by merely letting it stand uncovered). One of the reasons I thought of n-butylamine and
butyraldehyde is that their boiling points are not too far off from that of the ethanol/water azeotrope. Anyway, the distillation has been a slow
enough process that there has been plenty of time and opportunity for low-boiling components to gas off, and the product is still contaminated.
I'm currently letting it sit with activated carbon (and occasionally shaking). I think I'll try the sodium carbonate that aonomus suggests, and then
redistill.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
What are you guys fermenting? I've made EtOH a couple of times by fermenting cane sugar or corn syrup and the distillate never smelled like
anything but EtOH. Having grown up in moonshining territory I'm very puzzled by what you're describing. Is your mash becoming contaminated with
air-borne bacteria that are making these impurities?? Instead of trying to remove the junk, figure out what's wrong and fix it.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
I fermented cane sugar, but my process was quite sloppy (I had falsely assumed that distillation would allow me to easily purify the ethanol).
Notably,
- rather than use a proper airtight container with one-way valve to allow CO2 to escape, I just used a heavy flat lid over a bucket. While the amount
of air this lets in is not enormous I'm sure it allowed for some oxidation (to aldehyde or w/e).
- I didn't filter or flocculate the fermented liquid, just let the yeast settle for a couple of weeks and then scooped out liquid to put in my RBF.
One consequence of this is that during distillation I'm also cooking some organic stuff (yeast); in fact the remaining organic matter forms little
white globs in the boiling liquid.
Obviously a little more attention to the proper way of doing things would probably go a long way in eliminating these problems. But I'd still like to
salvage the two liters of contaminated EtOH I've made :-)
(edit: also, temperature control during fermentation was wonky; the room was too cold, so I had to use a heater, but once the fermentation was going
full bore that was a little on the hot side... and so on... there were swings...)
[Edited on 10-3-2010 by bbartlog]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by bbartlog  | I fermented cane sugar, but my process was quite sloppy (I had falsely assumed that distillation would allow me to easily purify the ethanol).
Notably,
- rather than use a proper airtight container with one-way valve to allow CO2 to escape, I just used a heavy flat lid over a bucket. While the amount
of air this lets in is not enormous I'm sure it allowed for some oxidation (to aldehyde or w/e).
- I didn't filter or flocculate the fermented liquid, just let the yeast settle for a couple of weeks and then scooped out liquid to put in my RBF.
One consequence of this is that during distillation I'm also cooking some organic stuff (yeast); in fact the remaining organic matter forms little
white globs in the boiling liquid.
Obviously a little more attention to the proper way of doing things would probably go a long way in eliminating these problems. But I'd still like to
salvage the two liters of contaminated EtOH I've made :-) |
Yes, those two things should be fixed. The one
way valve can just be a tube leading under a little H2O to make a bubbler. I wouldn't let it fester for two weeks it. It becomes a putrefication
instead of a fermentation.
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
Aye, you won't ever fix your wash with sodium carb. if you didn't do it air tight and as sanitary as possible. I bleach rinse then wash for 20 minutes
with flowing water to get rid of all the chlorine traces. Any other bacteria or mould growing in the fermenter will cause all those odors.
You *need* to do it oxygen-free, otherwise oxidative respiration to acetic acid will occur.
Also, temperature matters, if you let it heat up too much, the yeast will end up making off-flavours.
Finally, you need to filter off as much of the yeast as possible, once you boil them they will lyse and release all sorts of nasty flavours. Also
carbon will remove most odour causing agents, but if you use any sort of SO2 releasing compound to kill off the yeast, you need copper in the vapour
path of the distillation rig, lest you get lots of SO2 condensing back in with your distillate.
I will be honest, I did do a bit of overkill for filtration. I used a fining agent (sililic acid and chitosan (basically charged species which cause
the cells to aggregate then precipitate out as a thick goop at the bottom), followed by careful filtering over lots of diatomaceous earth. It took a
week to filter bit by bit under vacuum filtration, but the clarity meant that no yeast would be making the distillate contain off-smells.
Funny that I think I might have been able to buy taxed alcohol at the price factoring in all the water wasted from the water-aspirator.
|
|
|
rrkss
Hazard to Others
  
Posts: 193
Registered: 18-12-2009
Member Is Offline
Mood: No Mood
|
|
I've always done small batches with plain old white sugar. I do 8 liter batches at a time. Add 1.45 kg of granulated sugar to 6 liters of water.
Then I add 2 tablespoons of KNO3, K2HPO4, and MgSO4 to the wash. After I mix everything up, I take 1 liter of this sugarwater and put it in an
erlenmeyer on a stirplate. I add my yeast and run the stirplate for 18 hours on high creating a nice little vortex. This grows I nice starter which
I add to the rest of my sugar water wash. The key to the starter is to maximize aerobic respiration of the yeast growing as massive of a colony as
possible.
In about 7 days I get my 14% ethanol water mix which I distill. My product usually smells slightly yeasty when I finish the first distillation but
after drying on the molecular sieves and redistilling it smells like ethanol.
I usually get around 550 mL of anhydrous 200 proof ethanol doing this.
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
| Quote: | | once you boil them they will lyse and release all sorts of nasty flavours |
I actually think this is the biggest factor inasfar as the fermented product smells pretty good *prior* to the first distillation (though not to the
point that I feel like drinking a glass...). I did sterilize the bucket prior to use, and while the lid setup was somewhat primitive the combination
of what amounted to a wet plastic gasket and internal CO2 overpressure means that not much oxygen (or other organisms) got in until later, when the
fermentation had slowed. And acetaldehyde and acetic acid by themselves (while surely undesirable to someone who wants to consume the product
directly) are things that a decent column should be able to separate from the ethanol.
| Quote: | | It took a week to filter bit by bit under vacuum filtration |
Ouch. I actually did attempt a quick filtration (through three inches of fine sand over a cloth) but the yeast is too tiny to be removed this way,
barring some sort of prior treatment to flocculate it. Anyway it takes me long enough to distill the stuff that I'd be loath to add on another week of
processing to the project.
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
I think that its not neccessarily size-exclusion filtration that is going on, but charge affinity filtration of some sort since even scraping off the
top layer of diatomaceous earth/cells isn't enough to 'renew' a plug of diatomaceous earth. Cells are physically sticking to the diatomaceous earth,
and thats why it works so well compared to just filtering by a solid support. I got the stuff from a fish/aquarium store pretty cheap too.
Another thought struck me, why could we not use continuous centrifugation to filter out cells from the wash? People that take waste vegetable oil
filled with all sorts of carbonized particulate use high speed home-made centrifuges (metal bowls bolted onto wood working routers essentially) and
pull out all the stuff that filtration through a mesh wouldn't be able to...
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
Filtration seem to be a pain... At the moment I am making a project of beer production for chemistry at school. Before to start our beer, we
have make a visit to a micro brewer. They are use to filter the beer and they use a kind of filter with compress plate of metal with groove
inside...don't remember the name... Because this thing coss a lot, they suggest that we use a type of polymer (ISINGLASS) that make all the yeast and
other particule to coagulate and fall to the bottom.
[Edited on 10-3-2010 by Bikemaster]
|
|
|
trilobite
Hazard to Others
  
Posts: 152
Registered: 25-2-2004
Location: The Palaeozoic Ocean
Member Is Offline
Mood: lonely
|
|
Laboratory-Size Continuous Distillation Unit
Ind. Eng. Chem., 33, 1455-1459 (1941)
Attachment: Ind_Eng_Chem_lab_cont_dist_unit.pdf (563kB)
This file has been downloaded 766 times
|
|
|
Ramiel
Vicious like a ferret
  
Posts: 484
Registered: 19-8-2002
Location: Room at the Back, Australia
Member Is Offline
Mood: Semi-demented
|
|
What about using bentonite for finning and filtration? Always worked for me.
Caveat Orator
|
|
|