| Pages:
1
2 |
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
But the (stored) energy required to effect rupture, if both bottles are identical, should be equal in either case.
The smaller gas-volume above the water would then, I think, need to be more highly compressed than that in the first container. . .
I'm just guessing!
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
No, because the liquid would be pressing on the glass the same amount as a gas would; so the same amount of pressure would burst both bottles.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
As I said, I'm just guessing here, but you're essentially saying that any small volume of compressed gas over any large volume of incompressible fluid
can rupture a container just as any large similarly compressed gas-volume can?
You may well be right, but how sure are you, MagicJigPipe?
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Thats not what he's saying, but that is also correct. The incompressible fluid is also pressing on the vessel, and when the failure pressure is
reached, a vessel containing only incompressible fluid will also rupture. Hydrostatic testing is preferred because if the vessel does rupture, then
the expansion of the incompressible fluid is minimal. However if a gas was present then it will vastly increase in volume as the pressure is relieved,
essentially exploding the vessel.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Thanks DJF90, the penny's dropped.
Note I wasn't trying to contradict anyone, despite how it looks.
You made me remember how ice can rupture a bottle without exploding.
It makes sense now. . .
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Might as well show a picture of the thing...
Here is the apparatus. Its not exotic or beautiful, its only purpose was to pressure test bottles of various sizes; so far it has been very
successful.
Warning: I hope anyone who attempts similar work exercises due caution. Always, pressure test bottles filled with water (at the temp
you wish to operate the device). Additionally, only operate experiments at half the highest tested pressure of the particular bottle. Finally,
always expect that the bottle will explode and plan accordingly.
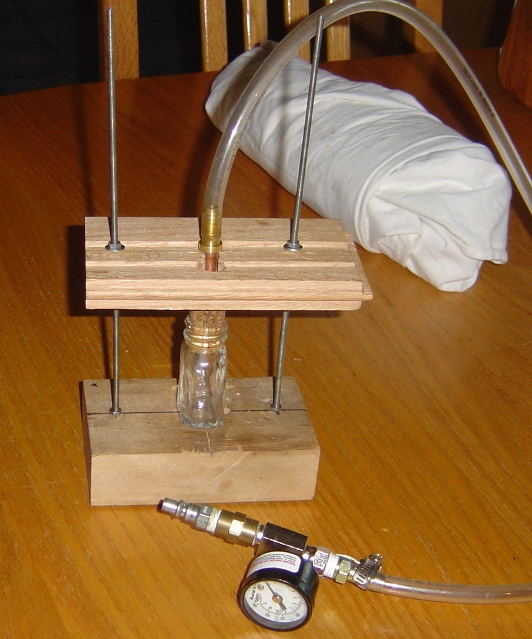

Things which have changed since the pictures were taken
*The tubing was replaced (after it yielded) w/ automotive fuel line for carburated vehicles, which is fairly cheep, tolerant of high temps and good
to ++200psi (at high temps).
*The natural cork was replaced with synthetic (the solid rubbery type, not the foamy w/ plastic veneer); the natural cork leaks appreciably at almost
any pressure, the synthetic did not leak.
Some details about construction
*4mm threaded rods; countersunk washered locknuts on bottom.
*Top piece is scrap oak wood flooring; it has a fancy notch cut into it (somewhat visible in both pictures) to allow the barb to fit through while
still maintaining good contact w/ cork
*The stopper piece consists of a hose barb soldered to a piece of 3/8 inch (IIRC) copper tubing which has a US nickel drilled and soldered to it (the
idea is the pressure pushes the stopper against the nickel to create a better seal; no idea if this helps at all).
*The gauge you see in the picture has a valve obscured from view which is nice for slowly filling the bottle.
Things I would change
*I would use the thin (but very strong) oak flooring for the bottom piece, as magnetic stirring is weakish through the thick piece of wood.
*I should have drilled through both the top and bottom pieces at the same time so they lined up a little better (not a big deal though)
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
nitric
Harmless

Posts: 40
Registered: 18-8-2008
Member Is Offline
Mood: nitrous
|
|
ive had a experience when i was younger with trying to make a hydrogen torch( idiotic to the max  ) with a peizoelectric sparker, peice of lithium, and a glass funnel. i put the peice of lithium in the bowl of water
and quickly covered it with the funnel, then with a face mask and welding gloves, put the sparker end of the peizo to the flow spot of the funnel and
sparked it. The funnel flashed up, then exploded with a deafening noise ) with a peizoelectric sparker, peice of lithium, and a glass funnel. i put the peice of lithium in the bowl of water
and quickly covered it with the funnel, then with a face mask and welding gloves, put the sparker end of the peizo to the flow spot of the funnel and
sparked it. The funnel flashed up, then exploded with a deafening noise  .
after that i was in shell shock for about a week then back to normal. .
after that i was in shell shock for about a week then back to normal.
|
|
|
| Pages:
1
2 |