ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
I bought a Mass Spec!
Hi everyone...
Bit of an interesting story here... I know I am not on here as much as I used to be, and my YouTube and Website are both inactive, too. Truth is, life
took over, but now that I am almost done with school I will have more time for my own chemistry instead of my PI's.
I am a HUGE fan of Waters instruments. There is no doubt that a Waters UPLC or MS is the most beautifully engineered workhorse of any analytical lab.
However, when you plop one down with a bunch of organic chemists, shit happens. That is how I spent the last 3 years of my life becoming a covert
expert in repairing LCMS systems: especially Waters.
I just so happened to be at a property disposition auction last week when a nice ZQ- single quadrupole, ESI source- came to my attention. I was just
informed that my bid of ONLY a couple hundred dollars won. The biggest challenge is going to be lifting this 200 pound beast into my Ford Fusion.
After that will come the fun part. I plan on hooking it up to my HPLC to have my very own LCMS system. The only things I will need are a nice vacuum
pump and a tank of nitrogen. The vacuum will be a bit tricky: I don't think a Harbor Freight boi would make an adequate roughing pump. Nitrogen is
easy. Of course there is the chance that it could be broken, but that wouldn't be too hard for me.
Now, a single quad isn't anything compared to the TOFs that I work with on a daily basis, but it would still be a nice tool in my lab, and after I get
it running, I would be happy to run samples for any of the regular members on this website. I am going to go pick it up tomorrow, so I will be sure to
keep you all updated on the status of my new adventure: amateur mass spectrometry!
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Nice! As someone unfamiliar with these systems, how much of a given sample would be needed to do a proper test? Is it possible to test chirality of
enantiomers? And presuming a member did send you a sample or two, what would be the best way to package it?
I occasionally purchase chemicals where the in-house analysis paperwork seems doctored, and it would be nice to have some way to dispute reagents of
questionable purity.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
One milligram of sample should be enough to do as many tests as I want. A typical microliter injection should contain roughly one nanogram of
analyte... so it is like dissolving a milligram in a milliliter of solvent and diluting a thousand times. If you can see any color in the vial,
9999/10000 times it is too concentrated. But for some reason you can't tell that to organic chemists, who carelessly inject concentrated shit and
dirty the source.
Testing chirality? Yes and no. For example, if you put D-glucose and L-glucose in the mass spec you would only see one parent peak since they have the
same exact mass. However, you can attach a metal-reference-base ligand, inject the complex via ESI and determine the chirality based on what part of
the complex cleaves the most. It is difficult, but it can be done.
There are a lot of easy ways you could send a sample. Since I only need less than a milligram, there is no need to send large bottles or vials. A tiny
vial in a bubble shipper would be adequate for liquids and solids alike. For nonhazardous solids, a milligram 'crumb' folded in aluminum foil or paper
would be fine.
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Mass spec is a great technology, obtaining one for a few hundred dollars and knowing how to handle it (expertwise as I understand) is great! Mass
specs are not the easiest machines!
I worked with a guy who did transcriptome 12C/13C MS for me, but I always had to outsource it to him.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Wow, this could potentially be a great resource for amateur chemists. I'm very impressed.
Quote: Originally posted by ScienceHideout  | If you can see any color in the vial, 9999/10000 times it is too concentrated. But for some reason you can't tell that to organic chemists, who
carelessly inject concentrated shit and dirty the source.
|
There was this one grad student in the lab where I worked as an undergrad, who was notorious for clogging up the GCMS. I wouldn't be surprised if he
just injected crude reaction mixture straight into the thing.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Quote: Originally posted by ScienceHideout  |
Testing chirality? Yes and no. For example, if you put D-glucose and L-glucose in the mass spec you would only see one parent peak since they have the
same exact mass. However, you can attach a metal-reference-base ligand, inject the complex via ESI and determine the chirality based on what part of
the complex cleaves the most. It is difficult, but it can be done.
|
If you hooked it up to your HPLC then you could also get a column with chiral stationary phase.
|
|
|
Steam
Hazard to Others
  
Posts: 238
Registered: 25-3-2014
Location: Minnesota
Member Is Offline
Mood: Triple Point
|
|
Let us know when you get it up and running, I would be interested in having some samples run.
DISCLAIMER: The information in this post is provided for general informational purposes only and may not reflect the current law in your jurisdiction.
No information contained in this post should be construed as legal advice from the individual author, nor is it intended to be a substitute for legal
counsel on any subject matter. No reader of this post should act or refrain from acting on the basis of any information included in, or accessible
through, this post without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from a lawyer
licensed in the recipient’s state, country or other appropriate licensing jurisdiction.
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
I could, in theory but I have run chiral columns before and it seems to me that chiral columns are much more sensitive to impurities in the solvent
bottles (or the sample for that matter). They also have some interesting Van Deemter mass transfer resistance, so efficiency falls off a cliff if you
run it just a little too quick. Temperature also effects chiral columns much more than usual.
Not to mention they are three times the price as normal columns.
Most of my experience is in sugar chemistry, where it is safe to assume that pretty much everything is D except rhamnose. I can understand how chiral
columns may be useful for organic chemists interested in mechanisms, but if someone just wants to verify they have L-tryptophan instead of
D-tryptophan, a simple guess would be just as accurate.
Melgar: What application are you specifically referring to where you need to know the enantiomer's absolute config?
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Probably just to test how well I did some resolution procedure. For instance, getting DLPA (d/l phenylalanine) and separating the enantiomers. It's
probably not that important, but I was looking into it recently and was just curious.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
Quote: Originally posted by ScienceHideout  |
I just so happened to be at a property disposition auction last week when a nice ZQ- single quadrupole, ESI source- came to my attention. I was just
informed that my bid of ONLY a couple hundred dollars won. |
Awesome, gratz - I hope you enjoy it and get it up and running. :-)
\"It\'s a man\'s obligation to stick his boneration in a women\'s separation; this sort of penetration will increase the population of the younger
generation\" - Eric Cartman
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Quote: Originally posted by ScienceHideout  |
I could, in theory but I have run chiral columns before and it seems to me that chiral columns are much more sensitive to impurities in the solvent
bottles (or the sample for that matter). They also have some interesting Van Deemter mass transfer resistance, so efficiency falls off a cliff if you
run it just a little too quick. Temperature also effects chiral columns much more than usual.
Not to mention they are three times the price as normal columns.
Most of my experience is in sugar chemistry, where it is safe to assume that pretty much everything is D except rhamnose. I can understand how chiral
columns may be useful for organic chemists interested in mechanisms, but if someone just wants to verify they have L-tryptophan instead of
D-tryptophan, a simple guess would be just as accurate.
|
That's interesting. I'm still looking out for affordable chiral columns. Currently I only have C18 columns.
Re mobile phase purity: I hope you have easy access to ultrapure water and to purchase HPLC grade solvents...
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
@Melgar: I routinely use the fixed-ligand kinetic method to identify relative confirmations of sugars. The procedure was created by Nikki Pohl at
Indiana University. Nice lady. The interesting part is that, even though the result is always D in my lab, it also provides the D/L configuration as a
consequence of how it works. I'm sure it can be adopted to amino acids quite easily.
@DavidJR: I bought a MilliQ water purifier from property disposition a while back for 25 bucks. To my surprise the resins are less than a year old! As
far as methanol or acetonitrile, it isn't uncommon for me to ask my PI if he'd let me buy something personal from the stock room. Usually he doesn't
even make me pay him back because I'm always saving him money fixing his machines. I don't think he's on to me that I have a lab at home, since what I
ask for generally has uses outside the lab. I'm convinced he thinks I clean my windows with n-Propanol and put nonpolar stuff in my gas tank. If I
bought an HPLC solvent, it would catch his attention, but it is common for the stock room people to put the solvents in the wrong spot, and he'd
probably ignore it. Another guy in my lab has a fancy car and our PI always let's him take methanol for the injectors, lol. Our boss is cool.
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Quote: Originally posted by ScienceHideout  |
I bought a MilliQ water purifier from property disposition a while back for 25 bucks. To my surprise the resins are less than a year old!
|
That's a good deal. I got my Milli-Q for £60 excluding the three consumable cartridges. I bought those on eBay as new-old-stock, still sealed in
original packaging, so I'm sure they're fresh. According to the display I get 18.2Mohm cm and 3ppb TOC @25C. I feed mine from a reservoir that I fill
manually every now and then with water from my dirt cheap eBay reverse osmosis/mixed bed DI system. I have a couple of minor issues though:
First, it complains with an error code sometimes ("A10 error 4") which according to the manual is caused by the intake water temperature being <=
4C. I guess they don't really plan for these machines to be located in a cold garage... Fortunately, it doesn't seem to actually affect the product
water quality. I'm slightly concerned about the possibility of damage from freezing during the colder months. I suppose that's mitigated to some
degree by the automatic periodic water recirculation.
Second, the model I have (Synthesis A10) has a (non-consumable) ultrafiltration membrane, for removal of pyrogens I believe (no concern for my use).
Consequently it must be connected to a drain (or waste container) as it periodically flushes the 'crud side' of the membrane. Additionally you are
supposed to run a monthly sanitisation procedure involving putting 3 grams of sodium hydroxide in a hole on the top of the machine and having it run a
cleaning program that lasts 7 hours. However: during both normal operation and sanitisation, very little water escapes from the UF reject line on my
unit - much less than is supposed to. I think that perhaps there may be a blockage somewhere or a failed solenoid valve. This would probably explain
why the product water flow rate is a bit lower than the manual specification - the membrane is probably somewhat plugged up due to the cleaning being
ineffective.
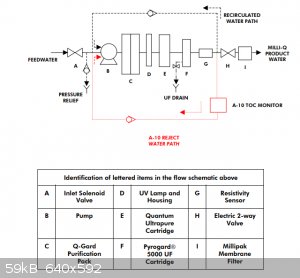
Quote: Originally posted by ScienceHideout  |
As far as methanol or acetonitrile, it isn't uncommon for me to ask my PI if he'd let me buy something personal from the stock room. Usually he
doesn't even make me pay him back because I'm always saving him money fixing his machines. I don't think he's on to me that I have a lab at home,
since what I ask for generally has uses outside the lab. I'm convinced he thinks I clean my windows with n-Propanol and put nonpolar stuff in my gas
tank. If I bought an HPLC solvent, it would catch his attention, but it is common for the stock room people to put the solvents in the wrong spot, and
he'd probably ignore it. Another guy in my lab has a fancy car and our PI always let's him take methanol for the injectors, lol. Our boss is cool.
|
Convenient arrangement. Are you specifically trying to keep it a secret from your coworkers?
[Edited on 21-11-2018 by DavidJR]
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
David, mine doesn't reject much water either during the cleaning cycle, but it does during the air purge. Maybe it isn't supposed to reject much water
in the cleaning cycle?
It's no secret that I'm allowed to take solvents home with my PI's permission, but I don't advertise that I have a home lab. Obviously I'm not doing
anything bad...I just don't want to have to answer questions to everyone in the department. It is still a pretty bizarre hobby compared to cooking or
woodworking.
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Texium
Administrator
       
Posts: 4579
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Congratulations on this excellent find! Mass spec is amazing. I only got into it more recently, because up until a couple months ago, we had an
incompetent lab tech who ran the machine, and would always inject way too much into it and then tell me that my product isn't pure. So I didn't really
trust it when it was in his care. NMR is more useful for what I'm doing anyway. After he left though, my coworkers and I figured out how to
actually use the machine ourselves, and ever since then it's been great! Now I want to use it for everything. Having one at home would be
remarkable, though I'd be nervous about the cost of upkeep and keeping it evacuated.
Also, if your PI is as cool as you make him sound, you should totally tell him about your home lab! I told mine right from the beginning, and it's
only worked in my favor. He even offered me some nice glassware that we didn't need anymore due to downsizing. If they know you're passionate enough
about chemistry to pursue it in your free time, that'll set you apart and might open up some opportunities that a "regular" student wouldn't be able
to access.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Quote: Originally posted by ScienceHideout  | David, mine doesn't reject much water either during the cleaning cycle, but it does during the air purge. Maybe it isn't supposed to reject much water
in the cleaning cycle?
|
Well, what made me think that it's supposed to reject a lot more water is this statement in the manual: "You will need a minimum of a full 30 L
Reservoir fed at 3 LPH".
I have a 25L reservoir with no automatic feeding. The cleaning process barely uses a couple of litres. It seems very odd that they'd specify 30L +
3L/h if it isn't expected to use a lot of that.
I don't recall whether or not much water came out of the UF reject port during the air purge. I'll try doing another air purge when I get home tonight
and I'll report back.
Quote: Originally posted by ScienceHideout  |
It's no secret that I'm allowed to take solvents home with my PI's permission, but I don't advertise that I have a home lab. Obviously I'm not doing
anything bad...I just don't want to have to answer questions to everyone in the department. It is still a pretty bizarre hobby compared to cooking or
woodworking. |
Unusual perhaps, but I don't think it's bizarre. I studied Electronics & Software Engineering (though I wish I'd studied chemistry...) and quite a
lot of my fellow students did programming or electronics design 'for fun' in their own time. Chemistry at home is a bit less accessible, but I don't
think it's any more bizarre a hobby to have.
[Edited on 21-11-2018 by DavidJR]
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Quote: Originally posted by DavidJR  |
I don't recall whether or not much water came out of the UF reject port during the air purge. I'll try doing another air purge when I get home tonight
and I'll report back.
|
Ok, just tested this. During a single 5-minute air purge cycle, 2.4 litres of water was dispensed from the point-of-use dispenser. No water came out
of the UF reject port until the very end when the pump clicked off - a single spurt of water measuring 7ml.
I think something is not right here.
Also, the container I usually have on the UF reject line is 1L in capacity and I only have to empty it around once every week.
[Edited on 21-11-2018 by DavidJR]
I think I know what the problem is........... taking the cover off revealed that although the ultrafiltration cartridge is present, it has been
bypassed completely by a previous owner...
[Edited on 21-11-2018 by DavidJR]
Tried to reconnect the Pyrogard cartridge, and the push fitting on the reject port is leaking quite badly. I think perhaps the previous owner
disconnected it for that reason alone - the cartridge itself might be OK if I can replace the fitting
[Edited on 21-11-2018 by DavidJR]
Tightened up the fitting and tried again. It still leaks but not quite the deluge I had before. Unfortunately the resistivitity of the product water
dropped off a cliff! I think I'll put it back how I found it and assume that the previous owner probably had a good reason to bypass it. I'll stop
doing the sanitisation process too as all it's going to do is waste my ion exchange resin.
[Edited on 21-11-2018 by DavidJR]
Re-bypassed the UF cartridge and I'm back to on-spec product water.
With the way the plumbing is, the small squirt of water i was getting out must not have come from the UF reject port, but from the overpressure relief
port. I had them plumbed together into the same waste hose. I assume that the machine closing a solenoid valve at a certain time causes a pressure
spike due to water hammer (and the software not knowing about the plumbing mod).
[Edited on 21-11-2018 by DavidJR]
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Spectrometrist's Log: Entry 1
Thankfully, the spectrometer fit in my car and came home in one... erm… rather 3 pieces. The plastic source cover came off while we were lifting it
in my car, and the top cover was missing screws. A quick once-around revealed that the overall condition of the instrument is, well, fair. I wasn't
expecting to get a perfect one for less than $500, or even for $5,000 for that matter. I believe that this instrument can comfortably detect masses up
to the 4 kDa range. More than acceptable for the amateur.
I have to say, I feel quite accomplished that I was able to lift it out of my car by myself. 200 lbs is 110% my body weight and I don't have time to
pump iron anymore, but I guess pulling something cool out of your trunk is sort of like mothers lifting cars off of their babies, you know?
Still, there is much to do. Waters instruments run on MassLynx or Empower. I have neither. I could probably get a copy since I know a great deal of
people at Waters, or maybe I could find some open source software that I can use. Other than that, a transformer to get it to run on 110V mains, a
tank of N2, and a nice vacuum pump (the biggest challenge) and I should have it running.
The very first order of business, though, as you can see, it looks like the source has never been cleaned. That, or they didn't clean it well. The
capillary even fell out of the nebulizer probe! Luckily, I have taken ESI sources apart before, so there is nothing stopping me today!
Note to self and others: If anyone references my log in the future to learn how to maintain a MS, please be careful and WEAR GLOVES! I don't know what
type of lab this instrument came from, and God knows what was injected into that source. For all I know, the chemist who owned this before could have
done a total synthesis of Batrachotoxin. I am not taking my chances. The components inside this source will never, ever, ever come in contact with my
bare skin. Ever.
 
P.S. @David, during an air purge, mine usually dispenses well over a liter. But I don't have the A10. However, my advice: as long as you are getting
good quality water out, I wouldn't worry about the mechanics of it.
[Edited on 21-11-2018 by ScienceHideout]
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Spectrometrist's Log: Entry 2
What's up guys,
Sorry for posting twice but I am quite excited about my new adventures and wanted to bring you along, too.
I decided to pull off the source housing so I could give it a thorough cleaning, until it became evident that I should just take the whole source out.
I'm glad I did, because I noticed a few things. Number 1: I found a dead bug in the ion guide. That was probably an interesting adventure for the
little guy, but he is out now. Hopefully he didn't bring any of his friends along who found eternal rest in the turbomolecular pump. Second discovery:
whoever the last dimwit was to clean the source components seemed to have forgotten the O-ring that slides onto the aperture between the guide and the
quadrupole housing... which is VERY important to retain good vacuum levels in each part of the instrument. I can just buy a new one for a couple
bucks, no biggie, but I am sure that caused a lot of pain to the lab that was using it last.
I know that mass speccy lingo is sort of strange, so I attached pictures of what I am doing.

Above: the mass spec source pulled to pieces.

Above: this is looking into the hole that the source mounts on. The chamber directly inside is under high vacuum, and it houses the ion guide, which
sits inside. The aperture fits in the hole at the end of the ion guide chamber. In that hole, you can see four out-of-focus shiny metal rods that form
a square. That is the quadrupole.

Above: These are some of the important parts I took out. #1 is the cone: the electrospray is directed orthogonally to this, and it acts like a vacuum
cleaner sucking the ions into a tiny hole. There are two levels to the cone, the outermost one spewing hot nitrogen which desolvates the ions. #2 is a
baffle, which prevents the spray from getting sucked in. #3 is the probe that delivers the solvent and molecules. They spray out of the capillary, the
metal wire tube that is sticking waaayyy too far out of the end, and nebulized with the gas line. #4 is the ion block, and #5 the all important ion
guide; the metal cup-looking thing on the end is the aperture.
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Spectrometrist's Log: Entry 3
Hey everyone, its been over a week since my last entry. I have come a long way with the mass spec.
Firstly, I shared the news with a professor in my department, who I am good friends with. She is famous for mass spec, and was tickled when I told her
I bought one. She also gave me a copy of MassLynx, which I am going to run on a $45 Windows XP laptop I got on good ol' Craigslist.
While I was talking with her, I mentioned the vacuum concerns I have. She said that the turbo will do pretty much all of the work, I just need to get
a simple rotary pump that operates within the specs of the turbo. When I looked up the turbo pump specs on Edwards, it looks like a Harbor Freight
pump would be excellent for this purpose. I hope it works well. I connected it with some garbage disposal parts I found, but I can't test the vacuum
until I have the instrument running.

As far as the power supply, since the instrument draws 220V instead of 115 mains, I ordered a transformer from Amazon. It should work fine with this.
The whole spectrometer uses a whopping 2 kW, so I figure it should cost somewhere around $0.30 to run for an hour, which isn't too bad if I decide to
operate it one or two days a week.
So, I cleaned all the source components using HPLC methanol- I got 4x4L for a total of $51. Each component was first soaked in formic acid/methanol,
then rinsed with pure methanol. The less fragile components, like the source housing, were scrubbed with steel wool to get off the stubborn spots. I
obviously didn't scrub anything else. The cone. The hexapole (which I found the O-ring it needs at Ace hardware). The block. Nothing.
    
I also re-seated the capillary into the probe.
If anyone has any questions about what stuff is or how I did something, please feel free to ask.
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Spectrometrist's Log: Entry 4
It lives!
Sorry, no pictures in this update.
Since the last update I didn't have much time, due to finals, but I did get a lot done.
The biggest problem that I ran into was getting the spectrometer to communicate with the computer. After much troubleshooting, I found that the
instrument will not establish an FTP server connection with the computer unless you name the username 'micromass...' how odd. But now they are
talking.
Powering up for the first time went smoothly, and I began pumping. This is where I ran into the problem: the Pirani gauge in the instrument indicates
1e-4 mbar on Masslynx when the vacuum is OFF. This is quite strange, and indicates to me that the gauge isn't communicating, since when you unplug it
the 1e-4 reading continues. The Pirani potential shows 0V. To be certain that it isn't the gauge itself, I took it to my professor friend and we
vented her instrument and switched them out. The gauge works fine. I think the Harbor Freight pump is pulling a decent enough rough vacuum, and the
turbo comes on but doesn't go past 11% speed. I took a physical look at the turbo and it seems to be fine.
The two possible sources of the problem that I came up with are: 1) the Mass Spec won't pump down successfully unless a vacuum pump is plugged into
the port on the back, and this somehow affects the Pirani gauge reading. 2) There is some issue on the circuit board...
I hope it is the first.
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
I have never worked on a Waters ZQ, but it will never run on a tiny vacuum pump like you are using. TheTurbo needs to be backed with a large pump such
as an Edwards E2M28, especially when you are running samples. The vacuum is not good enough, so the turbo never gets up to speed, even if it does get
up to speed, having a small pump will drastically shorten the life of the turbo! Usually these sort of instruments are backed by two of these large
Edwards pumps. I own 4 mass spec systems, but they are all GCMS based with EI and FAB sources, I need to buy an ESI instrument. The turbo really needs
a big pump to work right. I had a turbo die in a HP 5970 GCMS several years ago, I acquired a HP 5972, probably the best low res GCMS system,
diffusion pump based, no turbo problems. Turbos can be expensive! Don't kill your turbo! ESI will also require a nitrogen gas source such as a
cylinder, you will go through a lot of gas! Good luck, these instruments are nice to have, but can get very expensive. Not sure what sort of detector
your machine has, probably an electron multiplier, many of these don't play well sitting at atmospheric pressure...
[Edited on 17-3-2019 by benzylchloride1]
Amateur NMR spectroscopist
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
Hi BnCl,
Thank you for the advice. I know a lot of mass specs use quite large pumps: the ones I use at school typically EM40s or greater, if I am not mistaken,
but that is for a TOF. I figured that the Harbor Freight pump should *theoretically* be enough judging from the manual for the turbo pump, but having
got a new Pirani gauge and having determined that the old Pirani gauge was the source of the problem, I can definitely see that the vacuum pump is the
source of the problem while watching the diagnostics. Keeping what you posted in mind, I think that this week I am going to look for a new pump with
similar specs to the Edwards 28... something in the neighborhood of 21 CFM and micron-level ultimate pressure.
I also scored another API MS on eBay- this one a Finnigan- for $69 and shipping. I will post updates on this guy, too! 
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
A good quality 2 stage with the thicker 3m oil is the best.
I have had good repeatable 15 micron rating from the JB vacuum pumps for refrigeration systems, all so use metal pipes, thermoplastics let air diffuse
through too much.
I used copper and steel, so I just use break line connectors and fittings. and steel break line tubing (Inverted flares with a drap of vacuum oil to
lubricate the tightening)
Supco V45 gauge that was tested on a precision vac system to confirm calibration (It actually read down to .1 micron!)
[Edited on 31-3-2019 by XeonTheMGPony]
|
|
|
Texium
|
Thread Moved
29-11-2023 at 11:49 |