| Pages:
1
2
3 |
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Possible route to resorcinol from Tylenol / Paracetamol?
The only step I'm unsure about is how good the reductive demamination with EtOH is.

[Edited on 12/29/2007 by guy]
Edited title. Chemoleo
[Edited on 30-12-2007 by chemoleo]
Edit: No "e" at the end of Paracetamol
[Edited on 12/30/2007 by guy]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I'm not sure on the ration of the nitrate re OH and NHAc. The aceto group may be bulky enough to partially block the positions ortho to it.
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
I got the idea from this OrgSyn prep
http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3...
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
It is suggestive. On the other hand, the H on the HO- is a lot smaller than the Me on the MeO-; and while activating I beliieve RO- is less strong
than HO-
Related, but different, is this
http://jchemed.chem.wisc.edu/JCESoft/cca/cca5/MAIN/1ORGANIC/...
http://ci.nii.ac.jp/naid/110003627771/en/
that's suggestive that you may want to pre-treat the HNO3 with urea or such.
This might have some useful information
http://www.rsc.org/publishing/journals/AN/article.asp?doi=an...
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Hmm all your links give different info. The first link says you will get an o-nitro product while the second link says an m-nitro.
And the 3rd link seems to suggest nitration won't work at all for me.
If that 2nd link is right, it would be good news for me.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I don't see the acteanilide reduction happening the way you plan. The OS prep used a specific form of base called Claisen's alkali and I don't think
it's all that general in application .. something to read up on. The other ref's cited didn't show acetanilide being reduced by OH. So that's
another issue to reconcile. Obviously this is for fun since resorcinol is cheap enough.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I think you've mixed two steps:
Claisen's alkali i just an alcoholic solution of KOH, which often gives quicker, more complete hydrolysis of amides.
The reduction of a diazo compound by boiling alcohol is an old method. The main competing reactions are formation of the aromatic ether with the
alcohol, and the formation of the phenol from water and the diazo compound.
The Chemistry of the Diazo-compounds By John Cannell Cain
http://books.google.com/books?id=4ZE3AAAAMAAJ&pg=PA42&lpg=PA42&dq=diazo+reduction+(ethanol%7Calcohol)&source=web&ots=I_bY4_lErV&am
p;sig=Du7tLp_8LMDWJ0mOIfSgygoZOzI#PPA39,M1
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I was looking at the second step where he deacetylates with OH. I don't think OH is a strong enough base. Claisen's alkali is stronger, the alcohol
making some methoxide ions ...that was my main objection. I wonder if alcoholic KOH would do it?
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
So thanks to not_important, I revised the 1st step:
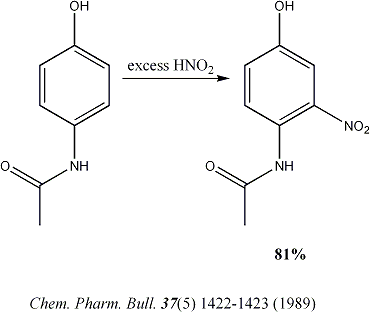
I trust this article more because they actually conducted 2 melting point tests and NMR to confirm the product.
[Edited on 12/30/2007 by guy]
I have a question: Will the nitro group ortho to the amino group make the reduction of the diazonium favorable even though there is an -OH group para
to it? I looked up the activating effects of substituents and the nitro group seems to be a stronger inducing group than the -OH group is activating.
[Edited on 12/30/2007 by guy]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Even if the nitrosation/oxidation gives you the correct regioisomere as the above reference clams (I have doubt's but could be), you will still have
to deaminate by nitrosating a p-hydroxyaniline which I do not think it is possible without ending with the corresponding p-benzoquinone (I
could be wrong though, but not motivated enough to check the literature).
PS: Resorcinol is terribly cheap and easy to buy. You might have at least chosen a better target. For example, if you manage to prepare
3-nitro-4-acetamido-phenol you could make 2-methyl-5-hydroxybenzimidazole from it... or anything at least remotely more interesting than resorcinol.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
I see. Well if you give some ideas on how to make 2-methyl-5-hydroxybenzimidazole I would rather try that.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
That was just an example meant to say that just about anything is more interesting than resorcinol. But if you are interested I will search the
literature for you when I'll have the time. In essence, you just reduce 3-nitro-4-acetamido-phenol to 3-amino-4-acetamido-phenol (with Na2S2O4, for
example) and then dehydrate to the corresponding benzimidazole (by refluxing in formic acid, for example). There are many other alternatives for both,
the reduction and the cyclization, but I don't know which reagents you have access to and what suits you better.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Other references for the nitration of paracetamol to 4-acetamido-3-nitrofenol (mp 221.5–222.5°C from H2O/EtOH):
Chemistry Letters, (2000) 48–49. (HNO3/CHCl3 in presence of ammonium molybdate, 87%)
Patent CN1966495 (HNO3, H2SO4 at <10°C, 2h, 76%)
Heterocycles, 43 (1996) 2495–2502. (nitration of O-acetyl-paracetamol followed by deprotection with K2CO3/MeOH)
Chemical & Pharmaceutical Bulletin, 51 (2003) 522–529. (first O-acetylation with Ac2O; then nitration with HNO3, AcOH; then
deprotection with NaOH)
References where 4-acetamido-3-aminofenol is described:
Journal of the American Chemical Society, 63 (1941) 1927-1929.
Chemical & Pharmaceutical Bulletin, 26 (1978) 1443-1452.
Patent US4518607
I also have several references for the cyclization of N-acetyl o-phenylenediamines to 2-methylbenzimidazoles, but unless you say you
need them, I will not loose time posting them.
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
THANKS A LOT!
I do want the references for 2-methylbenzimidazoles.
again thanks
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Examples of cyclizations of N-acetyl o-phenylenediamine to benzimidazoles {reference (substrate; conditions)}:
Synlett (2005) 340-342 (4-nitro-2-aminoacetanilide; heating in AcOH)
Tetrahedron, 23 (1967) 1863-1866 (1,4-diacetamido-2-aminonaphthalene; 1h reflux in AcOH)
Bioorganic & Medicinal Chemistry, 13 (2004) 175-184 (a substituted carbazole; 4h reflux in DMF)
Chemistry of Heterocyclic Compounds (Translation of Khimiya Geterotsiklicheskikh Soedinenii), 38 ( 2002) 793-794 (a polycyclic aryl;
3h heating in AcOH at 90°C)
Chemical & Pharmaceutical Bulletin, 50 (2002) 941-959 (2-amino-3-methoxy-4-carboxymethyl-6-chloroacetanilide; 1h reflux in toluene
in presence of TsOH)
Journal of Medicinal Chemistry, 45 (2002) 5813-5816 (N-(6-amino-1,3-dioxo-1H,3H-benzo[de]isochromen-5-yl)acetamide; 2 days reflux in
AcOH)
Tetrahedron, 57 (2001) 1793-1799 (N-acetyl o-phenylenediamine and another more complex substrate; heating in xylene/AcOH)
Journal of the American Chemical Society, 64 (1942) 1167-1173 (4-methyl-2-aminoacetanilide; treatment with HCl)
Journal of the American Chemical Society, 73 (1951) 4297-4299 (1,5-diacetamido-2-aminonaphthalene; treatment with HCl)
Indian Journal of Chemistry, Section B, 31B (1992) 177-182 (N-(6-aminoisoquinolin-5-yl)acetamide and others; treatment with HCl)
Chemica Scripta, 27 (1987) 269-271 (similar as above)
Khimiya Prirodnykh Soedinenii, (1983) 207-209 (2-amino-4-chloroacetanilide; another treatment with HCl)
Monatshefte fuer Chemie, 96 (1965) 614-624 (4,6-dibromo2-aminoacetanilide; 4h heating at 150-160°C)
Journal of the American Chemical Society, 67 (1945) 1074-1075 (1-acetamido-2-aminonaphthalene; heating for 20min at 225°C under
nitrogen atmosphere) References with various reactions on 5-hydroxy-2-methylbenzimidazole:
Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (1991) 1928-1931 (Friedel-Crafts acetylation on position 4 with
AcCl/AlCl3)
Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (1990) 1888-1892 (sulfonation on position 4)
Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (1990) 662-664 (p-nitrophenyl diazo coupling on position 4)
Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (1989) 2329-2332 (mono- and dinitration on position 4 and 4&6 with HNO3/H2SO4)
Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (1989), 1630-1636 (monochlorination on position 4 with SO2Cl2; dichlorination on
4&6 with HCl/H2O2; mono- and dibromination on position 4 and 4&6 with Br2)
Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, (1985) 1855-1858 (various aminomethylations on position 4)
5-hydroxy-2-methylbenzimidazole can also be synthesized directly from 3-amino-4-nitrophenol by reduction with SnCl2/AcOH (see Tetrahedron Letters,
46 (2005), 6741-6743).
PS: How is your nitration of paracetamol proceeding? Have you tried it out?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
wow thanks for your hard work Nicodem.
I haven't done the nitration yet until I've got all the information on the cyclization. I have all the materials necessary for the nitration.
I do not have glacial acetic acid or any concentrated forms of acetic acid...I assume it is just used to dehydrate the reaction between the amide and
the aniline. Could H2SO4 be used?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
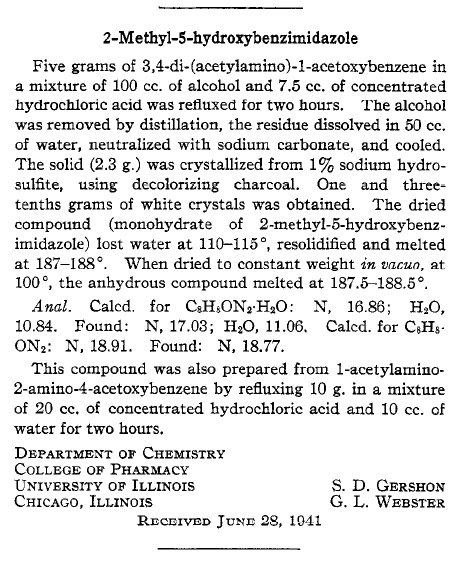
I would rather use HCl like it's done in several of the above examples. I managed to find a literature example that is nearest to what you are up to
(attached):
2-Methyl-5-hydroxybnzimidazole
S Gershon, G Webster
J. Am. Chem. Soc., 63 (1941) 2853-2853.
They cyclisize 4-acetyloxy-2-aminoacetanilide to 2-methyl-5-hydroxybnzimidazole with conc. HCl in ethanol which is just fine since your
4-hydroxy-2-aminoacetanilide is the first intermediate (phenyl acetates hydrolyze very rapidly).
Keep also in mind that 2-Methyl-5-hydroxybenzimidazole, like benzimidazoles in general, is a weak base and can form stable salts with strong acids
(pKa of protonated 2-methylbenzimidazole form is 6.3). The hydrochloride (mp 256°C), picrate (mp 217°C) and nitrate are described in the literature.
Due to the phenolic group it is also a weak acid (pKa of phenols is about 10, depending on the substituents).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Thanks I will try this experiment when I can
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
The nitration of p-acetamidophenol leads to tar formation, nitration with fuming nitric acid leads to a hypergolic reaction, flames and bad smell! I
would protect the hydroxyl group using ethyl bromide and sodium hydroxide to form the corresponding ethyl ether and proceed, 1st this will prevent
oxidation during the nitration, 2nd prevent oxidation during the diazotization and reduction. The hydroxyl group is then deprotected by refluxing with
concentrated hydrobromic acid as the final step of the synthesis.
Amateur NMR spectroscopist
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I can't figure out why anyone would using fuming nitric acid on such an activated substrate, except back in the early days of organic chemistry when
it was popular to "mix these together to see what happens" and which not infrequently was followed by "let's taste the product!" and that occasionally
resulting in "uuk...arrghh....gasp...uuuhh...thud".
Converting to a simple ether is overkill, popping the alkyl back off is bothersome at the least. Note that in the references Nicodem gave above, if
protection was mentioned it was O-acetylation which only needs alkali in water or alcohol to remove.
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
I tested the nitration on a very small scale several years ago. O-acetylation would certainly be eaiser then adding an ether group and then removing
it with strong acid. This was just a suggestion off the top of my head. Direct diazotization of p-aminophenol leads to a mess of quinone and other
unidentified products, so protection of the hydroxyl group would be mandatoryat least during the removal of the 4-amino group by diazotization and
reduction with ethyl alcohol.
[Edited on 19-6-2009 by benzylchloride1]
Amateur NMR spectroscopist
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Phenols are usually easy to nitrosate (for example by using NaNO2 in acetic acid at room temperature). Since the goal is to later on obtain the amino
group at that position, it really does not matter if you reduce a nitro or nitroso group - the end result is the same.
|
|
|
otonel
Hazard to Self
 
Posts: 84
Registered: 9-4-2005
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | Even if the nitrosation/oxidation gives you the correct regioisomere as the above reference clams (I have doubt's but could be), you will still have
to deaminate by nitrosating a p-hydroxyaniline which I do not think it is possible without ending with the corresponding p-benzoquinone (I
could be wrong though, but not motivated enough to check the literature).
PS: Resorcinol is terribly cheap and easy to buy. You might have at least chosen a better target. For example, if you manage to prepare
3-nitro-4-acetamido-phenol you could make 2-methyl-5-hydroxybenzimidazole from it... or anything at least remotely more interesting than resorcinol.
|
Where I fiind to buy pure resorcinol, what kind of product?
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Although this post is currently more relevant to the DDNP thread in the energetic forum I am posting here for two reasons, firstly I think this post
it nolonger quite relevant to the current state of the DDNP topic and secondly it was first described in this thread.
I recently started some experiment with the aim of producing benzene-ring substituted benzotriazoles. in this experiment the starting material was
paracetamol and the intermediate target was the 3-nitro-paracetamol via nitrosoation as described by Matsuno et al., Chem. Pharm. Bull. v37(5),
1422-1423; 1989.
The original reference by Matsuno et al states that paracetamol can be smoothly nitrated under weakly acid to neutral conditions by a three to five
fold excess of nitrous acid (27% yield) or by a mixture of nitrous acid and hydrogen peroxide (96%) but experimental details are at best sketchy.
Since the use of hydrogen peroxide presumably acts as an oxidizing agent there should be a lesser requirement for the nitrous acid and so in the
initial experiment described below I only used a threefold excess of nitrous acid. However, due to the presence of much tarry product and the
continued evolution of nitrogen oxides for many hours I feel that with hydrogen peroxide only a small excess, perhaps as little as 1.1 to 1.2 moles of
nitrous acid per mole of paracetamol, are required particularly given the mechanism proposed in Matsuno’s paper.
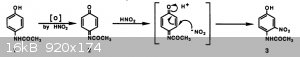
If the hydrogen peroxide brings about the initial oxidation to the quinimine then only one molar equivalence of nitrous acid is require. That said
when the hydrogen peroxide was added to the paracetamol no visible reaction was seen (I would expect the quinimine to be coloured). However, on the
addition of the nitrite an immediate orange colour developed, the reaction is slightly exothermic and appears very rapid.
Experimental.
3.006g of recrystallized (from tablets) paracetamol were dissolved in 20ml of warm 80% acetic acid and cooled to 5° C, 11.5 ml of 6% hydrogen
peroxide (1 mol. equiv.) were added and the mixture cooled in the ice chest to 0° C. 4.131g (3 mol. equiv.) of sodium nitrite were dissolved in 10ml
of water, diluted to 18ml and cooled to 0° C in the ice chest.
When removed from the ice chest after about 40 minutes the solution was at -7° C. The sodium nitrite solution was added to the paracetamol solution 2
ml at a time, after 4 ml had been added the reaction mixture had gone from colourless to dark orange brown and the temperature had risen to +7° C.
The reaction mixture was returned to the ice chest for about 25 minutes to drop the temperature to -5° C and the addition continued. Only a very
slight temperature rise now occurred and it is noticeable that almost immediately that a total of 6ml (1 mol. equiv.) of nitrite had been added the
solution began to effervesce giving off nitrogen oxide (brownish).
The reaction mixture was allowed to stand overnight to complete the reaction and warm up to room temperature, when a brown precipitate formed. 10ml of
40% NaOH was added to neutralise part of the acetic acid and the slurry and diluted to twice its volume with water and chilled in the fridge for 1
hour and then filtered. The cake was washed with a little (2-3ml) cold water and dried, the dried product weight 1.833g and was clearly a mixture of
two substances, one deep yellow spherical aggregates in a very dark brown amorphous material. A further 3ml of 40% NaOH solution were added to the
filtrate and the mixture extracted twice with 30ml quantities of ether. The ether was evaporated to leave a further 0.636g of orange coloured crystals
mixed with a reddish brown tarry material. The combine precipitate and extracts were mixed and recrystallized from 40ml of 75% aqueous methanol with
the addition of 0.2g of decolourising carbon. It was filtered through a preheated 42.5mm Buchner funnel and cooled to 5° C and the brownish felted
crystals recovered by filtration. Much material appears to remain in the filtrate and work on a better recovery method is ongoing. The filter cake was
dried overnight at 30° C and weighed 0.567g of orange brown prisms.
Discussion
Even with a threefold excess of sodium nitrite only about 4.3ml of 80% acetic acid are actually require by the reaction the remainder is merely
solvent. It may therefore be possible to reduce the overall volume and acetic acid concentration by adding a little methanol as a co-solvent and
reducing the acetic acid greatly to say 5ml or less. The dilution used above was used because only a very slight precipitate formed spontaneously,
probably because strong acetic acid is a good solvent for polar organic compounds. If this results in complete solution it would be advantageous to
use less and stronger hydrogen peroxide to minimise dilution of the reaction mixture. Furthermore the effervescence that occurs after the addition of
just 1 mol. Equiv. of sodium nitrite solution suggest to me that with hydrogen peroxide as an oxidant only one molar equiv. of sodium nitrite should
be required.
I plan to run several further experiments using only 5ml of 80% acetic acid 6ml or so of methanol and an appropriate volume of 12% hydrogen peroxide.
To these mixture I plan to add only about 1 to 2 molar equivalences of sodium nitrite as a near saturated aqueous solution.
A further issue that requires investigation is whether or not the use of a threefold excess of nitrite in the presence of hydrogen peroxide can give
rise to a dinitration product or just tarry products. From an examination of the various solid product it is clear that they are heterogeneous.
Work is also in progress on the best work-up protocol.
[Edited on 1-7-2015 by Boffis]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Maybe? you are assuming hydrogen peroxide is necessary for formation of the N-acetyl-p-benzoquinone imine intermediate, but that could come about by
oxidation by the nitrite. I think it is the excess of nitrite that drives the reaction to completion forming the 3-nitro-4-acetylaminophenol as the
ultimate main product, blowing right past the intermediates produced at lesser concentrations of nitrite as would exist under in vivo reaction
conditions of the stomach where the reaction proceeds only part way to completion, producing the intermediate benzoquinone instead.
Aqueous acetic acid at pH 4 is 0.00066 molar which is some really dilute vinegar.  so waving a pickle over the beaker while breathing across it gently might be enough to do it. so waving a pickle over the beaker while breathing across it gently might be enough to do it.
Seriously what may work fine is try an equimolar amount of acetic acid equivalent to the total NaNO2 plus maybe 2% in excess of theory needed to
convert all the NaNO2 to NaOAc and call that good.
I am guessing that is the adjusted pH for the reaction system with the sodium nitrite already added, since the sodium nitrite dissolves and forms a
distinctly basic solution, there must be a titration with acetic acid to lower the pH back to neutral and beyond that to a slight acidity at pH 4. It
would have to work that way I think and a sodium acetate / acetic acid buffer is being created in situ by that titration. They couldn't make it easy
and just state the quantities and volumes used. The maximum activity for the nitrosation is reported by others for similar reactions for the pH range
about 5 and I think pH rising to around 5.5 the activity quenches entirely, so the pH ranging 4 to 5 seems about right. It can maybe be a pH target
range for the pH maintained by buffering as is suggested for the use of a phosphate buffer as the alternative reaction system. I believe the lower pH
system at about pH 4 to maximum pH 5 would be a faster completing reaction with the higher yield.
If I understand the meaning correctly 7 moles of NaNO2 were used per 1 mole of paracetamol to obtain the 3-nitro-4-acetylaminophenol. 1 mole HNO2 is
needed to produce the N-acetyl-p-benzoquinone imine plus 1 more mole to convert the N-acetyl-p-benzoquinone imine to the 3-nitro-4-acetylaminophenol,
plus 5 moles NaNO2 excess was used, or 350% amount of theory of the 2 moles NaNO2 per 1 mole of acetaminophen. That 7 to 1 molar ratio works out as
3.2 grams NaNO2 per each 1 gram paracetamol for the nitration. 102% of the theoretical amount of acetic acid for neutralization of that Na value would
be 2.84 grams acetic acid per 3.2 grams NaNO2. The buffer calculations for the resulting byproduct sodium acetate solution will need to be done to
calculate the needed additional acetic acid for buffering the spent nitration mixture at the pH 4 or in the pH 4 to pH 5 range. That should put
things in the ball park for what reaction conditions the Japanese chemists neglected to provide details. Need to look at the solubilities and see what
seems reasonable for a reaction volume also.
The only way I can see the described reaction condition happening is to have a stirred slurry of paracetamol in an acetic acid / sodium acetate buffer
at pH 4, in an ice bath, to which is added in separate streams, equal drip rates of NaNO2 solution as reactant [A] and a calculated concentration and
equal volume of acetic acid as reactant [B] This scheme could maintain the pH 4 for the reaction as paracetamol in solution reacted with the 2
incoming reactant streams. As dissolved paracetamol is nitrated and drops out of solution, fresh paracetamol from the slurry will enter solution and
react, until the reaction is complete.
Aqueous acetic acid at pH 4 or a phosphate buffer solution at pH 7 is a kind of indefinite description of the reaction volume and reactant
concentration which would require some reverse engineering to solve that cryptic description. 
Here is the prior article which led to the article you reference and is reference 4
Formation of N-acetyl-p-benzoquinone imine, the well-known toxic metabolite of acetaminophen, by the reaction of acetaminophen with nitrite under
model stomach conditions.
Takafumi Ohta, Hiroaki Oribe, Manabu Ide, Shoji Takitani
https://www.jstage.jst.go.jp/article/cpb1958/36/11/36_11_463...
Abstract:
The main reaction product of acetaminophen (4mM) with nitrite (1mM) under model stomach conditions was identified as p-benzoquinone. HPLC analysis of
the reaction mixture with an electrochemical detector revealed the presence of N-acetyl-p-benzoquinone imine, the toxic metabolite of acetaminophen,
as an intermediate in p-benzoquinone formation. About 14% of acetaminophen was estimated to be transformed into this intermediate when the reaction
was carried out at pH 3 for 1 h at 37°C.
and here is the Journal page
https://www.jstage.jst.go.jp/browse/cpb/36/11/_contents?from...
Attachment: Chemical and Pharmaceutical Buletin Vol36 1988.pdf (501kB)
This file has been downloaded 652 times
What is going to be the potential "real bite" here is if the Japanese have misidentified a nitroso for a nitro.
3-nitroso instead of 3-nitro
would not make me a happy camper
If indeed it does turn out to be a 3-nitroso then it may possibly oxidize to the nitro by warming with dilute HNO3
[Edited on 7/2/2015 by Rosco Bodine]
|
|
|
| Pages:
1
2
3 |