# Load 2-5X the amount of DNA that would give bands of moderate intensity on an ethidium bromide stained gel. Typically this is something on the
order of 0.5-2.5 µg of a 1 kb fragment on a 30 ml 1% mini gel. These numbers are guess-timates so your milage may vary.
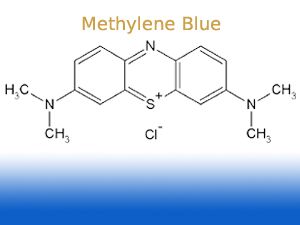
# Run the gel normally and then place in a 0.002% methylene blue (w/v, Sigma M-4159) solution in 0.1X TAE (0.004M Tris 0.0001 M EDTA) for 1-4 h at
room temp (22°C) or overnight at 4°C. Diffusion of the DNA does not seem to be a problem for fragments as small as 100 bp (3% Nusieve:1%agarose
gel). This avoids background issues associated with staining with 0.02% methylene blue for 30-60 min and then destaining for what seems to be forever
# If destaining is needed to increase the visibility of the bands place the gel in 0.1X TAE with gentle agitation changing the buffer every 30 - 60
min until you are satisfied with the degree of destaining. |