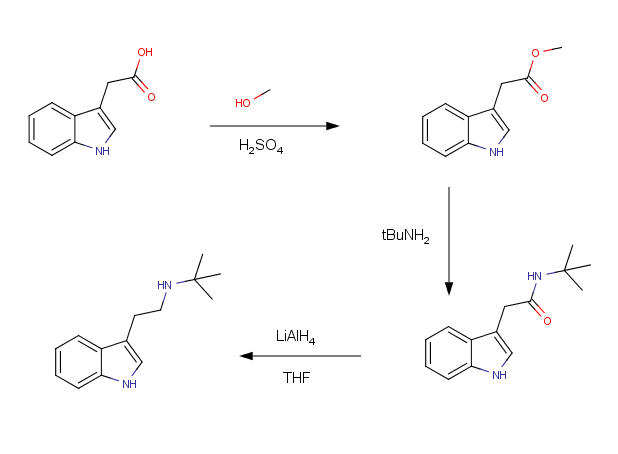
Message original : Sandmeyer
Eclectic,
He doesn't want to make t-butylamine, clearly, he has a starting material that he wants to use... But I agree that he shall use a different starting
material, I'd choose tryptophol, mezylate it and swap with t-butylamine...
[Edited on 16-5-2007 by Sandmeyer] |