Originally posted by mrjeffy321...
I just went out and tried a reaction using the ratios suggested by your chemical reaction.
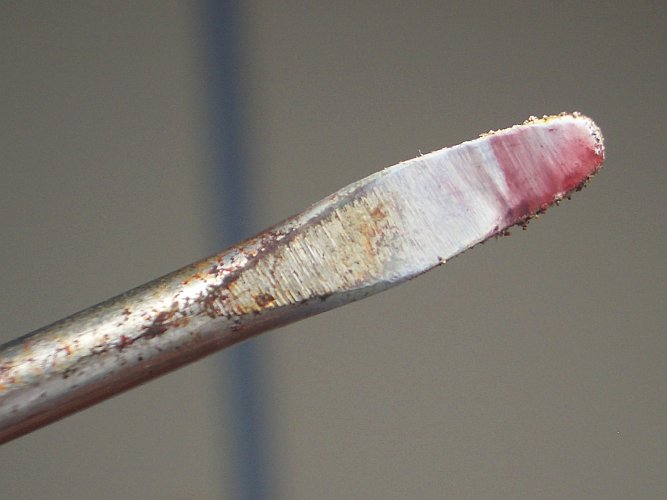
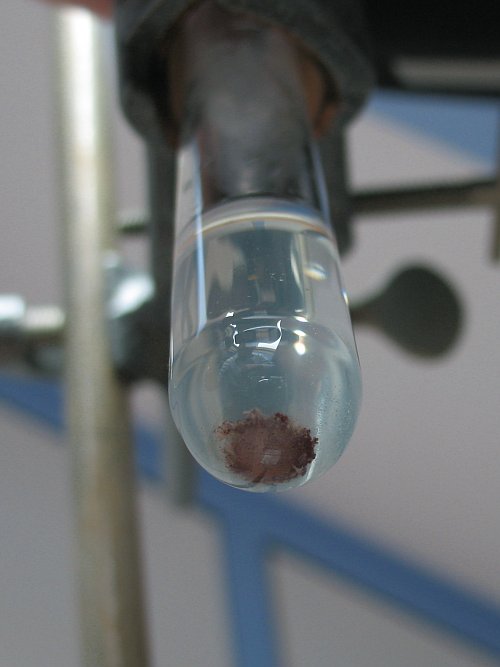
I used a strip of Mg ribbon to ignite the mixture. The first time, when the Mg burned down to the powder, nothing happened except the powder and its
container seemed to get very hot and change color from the gray-ish blue color originally to a much darker gray color. Upon inspection, the powder
had hardened into a solid, brittle, lump. I decided to try it again using the left-overs of the first try. I stuck another piece of Mg ribbon into
the hard lump. This time when the Mg burned down something did happen. I saw a small blue flame appear (something I did not see with the anhydrous
CuCl2 reaction) and a lot of white fumes were produced (not much, if any, color). This time, the remaining material left over in the container was
white. |


 <sub>2</sub>
<sub>2</sub> which heats up and heat helps speed things along
which heats up and heat helps speed things along