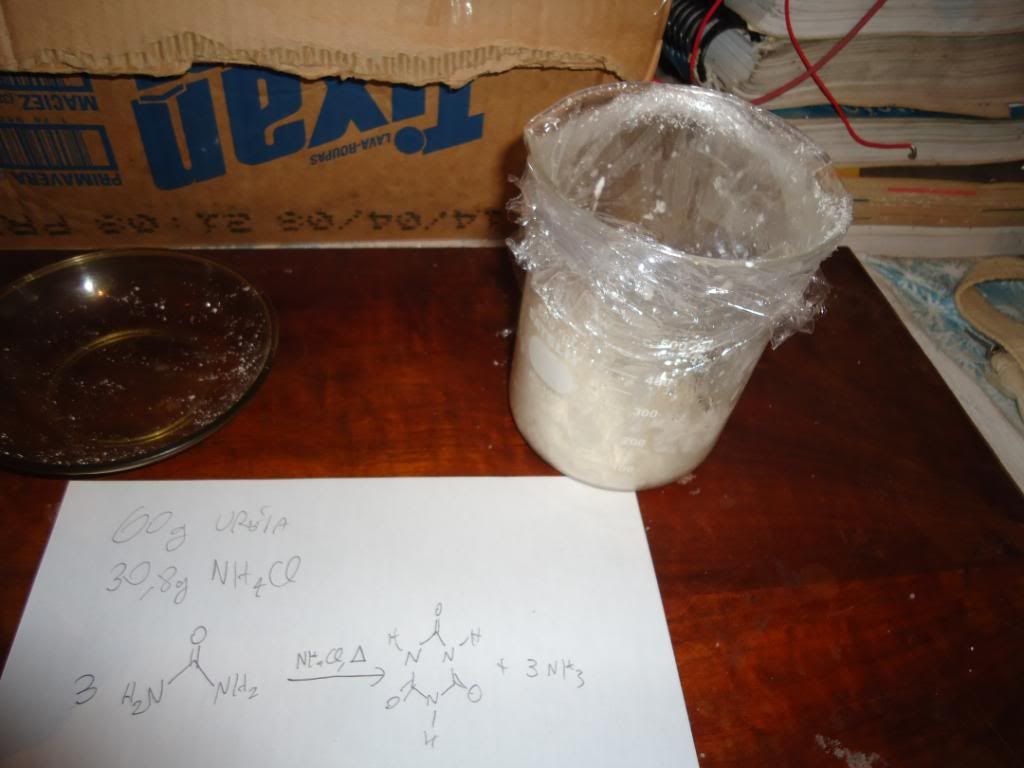This is one for the books it is so easy .
Put urea and ammonium chloride ( 1 to 0.56 molar ratio )
in a beaker and heat to melting , 15 minutes at 250C gives ~85% yield of cyanuric acid ( based on urea ) when the reaction mixture is diluted with H2O and boiled , the cyanuric acid dihydrate crystallizing out on cooling .
The reaction should be
3(NH2CONH2) -----> (HOCN)3 + 3 NH3
This would be a fine source for dry ammonia also .
The evaporated byproduct liquid left from the manufacture of urea nitrate from urea , ammonium nitrate and HCl contains
~90% ammonium chloride and this would probably be a
convenient source for that catalyst component .
[Edited on 21-3-2007 by Rosco Bodine]