
ARandomTroll5150 - 25-5-2017 at 11:18
I have been lurking on SM for a while now and could use a little help.
I wanted to make chlorates but decided to cancel that project for multiple reasons.
The graphite electrodes were already bought tough, so I thought about making sodium instead.
I want to make a downs cell like contraption for splitting NaCl into it's elements.
I would however like to use pure molten salt instead of mixtures with lower melting points.
The problem is that I can't think of any materials that can withstand 850°c, hot chlorine and molten salt.
I did a quick search and the only information I found on the chlorine collection hood/funnel material was in a dubious video on youtube. It was not
even in the patent (unless i missed it) 
I don't think that SS, glass or clay would work because their resistance is caused by oxides which would probably react with chlorine at 850°
if you can think of a material that is affordable and shapeable please let me know.
I also attached a crude drawing of the system (i suck at paint)
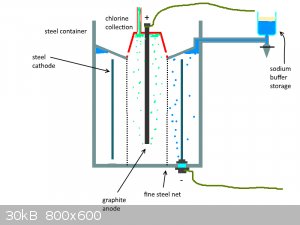
Magpie - 25-5-2017 at 13:03
Downs' patent specifies refractory lined iron.
You can find compatible materials for dry chlorine at the Cole-Parmer site:
https://www.coleparmer.com/Chemical-Resistance
You might be able to fabricate from steel pipe then line this with a castable furnace cement refractory. This is available cheaply at Ace Hardware.
Jstuyfzand - 25-5-2017 at 13:26
If you can get a good stainless steel cylinder somewhere and plug the one end with refractory cement (The end on the bottom which doesnt get contacted
with the chlorine) and stick the electrode through there, you are good.
Do not melt pure NaCl, mix with CaCl2 in a 33/66% Ratio NaCl/CaCl2 (Not exacty sure what the ratio was) and you have a mixture that melts at a much
lower temperature.
Buy a nichrome heating elements from ebay (Couple of bucks), coat the steel cylinder in sodium silicate and wind the element around it.
Rig it up to a PID controller. too high of a temperature and the sodium dissolves in the melt.
Alternatively, you can go the NaOH electrolysis route, heating it is easier because of the lower temperature, you can just use a glass fibre cloth
instead of the sodium silicate.
ARandomTroll5150 - 25-5-2017 at 13:53
Thank you. I will have to try copper for the funnel as it is one of the few materials that is accessible and can withstand the heat and chlorine. I
have to test it by passing chlorine through a heated tube and cutting it open to check for corrosion.

