
fluorescence - 28-1-2017 at 03:45
Hi,
So I'll have an exam in a few weeks on the lecture about asym. synthesis and there was this question in one of the older exams. I am not really sure
how to answer it properely.
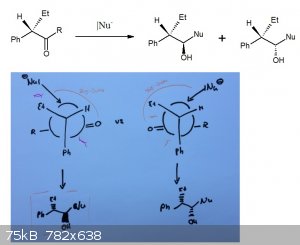
Usually, for the Felkin-Ahn model, you have the biggest group on the left side and then, pointing forward, always the group which is bigger than
Hydrogen to yield the Cram-product. Now this seems to be the other enantiomer and I'm a bit stuck which is now better.
So I drew the two possible ways and I think, if we assume R is a quite small group, the repulsion of the Carbonyle-Group with the Ethyl and Phenyl
rests is way bigger than the one the nucleophile has with the Ethyl rest, especially as the Bürgi-Dunitz trajectory can be a bit off the usual angle.
So I think the one where Et and OH are anti to each other is better and therefore the cram-product.
Any suggestions on the problem.
On the other hand I could also say the enantiomer to this one would obey the usual model and therefore be likely the one where they are syn to each
other and therefore this enantiomer must be syn as well but what's the point of such model if you can just look for a different molecule and twist it
till it works....
Sigmatropic - 29-1-2017 at 08:43
Even though this is my first post and I do not see a formulated question here are my two cents: I think you're missing that if the R group is quite
small the energy difference between the two conformations is low and hence stereoselectivity will be low.
So if we assume a large R group, I would not consider the repulsion between the ethyl or phenyl and the carbonyl but instead the repulsion between
Et/Ph and the R group. This suggests the newmann projection on the right is more favorable. Note that the trajectory of the nucleophile is unhindered
in the right projection. Thus I would pick the syn alcohol.
fluorescence - 31-1-2017 at 13:31
Thank you for your reply:
Yeah thinking about it (I think in the exam it was an -H where I put the R) it seems more like a syn-Produkt. I mean as mentioned above. If I chose
the other enantiomer as reactant I would automatically get the syn-product...so why should it be any different here, it's all just flipped but the
electronic and steric situations shouldn't change so it still remains syn just pointing into a different direction.
