
nitdhaval - 28-11-2016 at 20:49
I want to know about Ethylation of already prepared dye with Diethyl sulfate or Diethylcarbonate.
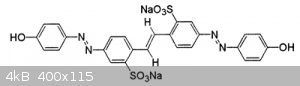
i have attached the structure of the same. which is best practice.
Our current route is via ethyl-chloride gas and spirit in an autoclave (6 ATM), which is very old process.
any suggestions.
DDTea - 28-11-2016 at 20:54
Where are you trying to ethylate? And what is your desired product?
Before we go about reinventing the wheel, tell us about the current process. How are yields and purity? What's wrong with it? Why are you
interested in other routes?
qeezur - 5-12-2016 at 12:18
Which functional group do u want to change here ?
I see two OH which can attack the CH2 of the CH3-CH2-OS(O)2O-CH2-CH3 in presence of triethylamine or pyridine. Another way can be to take ethyl halide
and convert it into methyl or paratoluene sulfonate then get this Nu attack done. Both will form an ethyl ether.
P.S. The lewis basic potency of aromatic OH /Phenol is lesser than usual OH because the nonbonding electrons on O will go into conjugation of ring.
