
xwonchem - 30-10-2006 at 05:36
Hi everybody!
does anyone know the method used to link two aromtic ring?
let me see the mechanism, reaction, conditions, and how to do it?
thanks.
Drunkguy - 30-10-2006 at 06:41
"Aryl iodides when heated with copper afford biaryls: this is the basis of the Ullmann reaction. Aryl copper intermediates are initially formed, but
the precise structures of these compounds are uncertain.
ArI -> [ArCu] -> Ar-Ar
The first step in the Ullmann reaction may be radical in nature, bu the second step is possibly a nucleophilic attack by [ArCu] on ArI.
solo - 30-10-2006 at 10:03
Reference Information
http://www.trincoll.edu/depts/chem/becmajfold/coollinks/micr...
Inducing All Steps of a Chemical Reaction with the Scanning Tunneling Microscope Tip: Towards Single Molecule Engineering
Saw-Wai Hla,1,2, * Ludwig Bartels,1, † Gerhard Meyer,1 and Karl-Heinz Rieder
PHYSICAL REVIEW LETTERS VOLUME 85, NUMBER 13, 2777, 2000
Abstract
All elementary steps of a chemical reaction have been successfully induced on individual molecules with a scanning tunneling microscope (STM) in a
controlled step-by-step manner utilizing a variety of manipulation techniques. The reaction steps involve the separation of iodine from iodobenzene by
using tunneling electrons, bringing together two resultant phenyls mechanically by lateral manipulation and, finally, their chemical association to
form a biphenyl molecule mediated by excitation with tunneling electrons. The procedures presented here constitute an important step towards the
assembly of individual molecules out of simple building blocks in situ on the atomic scale.
Attachment: Inducing All Steps of a Chemical Reaction with the Scanning Tunneling Microscope Tip.pdf (402kB)
This file has been downloaded 715 times
matei - 30-10-2006 at 12:03
Very useful reactions for the synthesis of biaryls are the palladium catalyzed cross-coupling reactions. Read this review:
http://rapidshare.com/files/1330911/Palladium-Catalyzed_Cros...
solo - 30-10-2006 at 12:23
Sometimes in the near future when someone researches this topic they will find the link to the excellent article not available, hence it's a good
idea to upload referenced articles so they can be available to future researchers......my humble opinion, hence the article..........solo
-------------------------------------------------------------------------------------------
Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds
Norio Miyaura' and Akira Suzuki'nt
Chem. Rev. 1995, 95,2457-2483
Contents
I. lntroduction
II. Svnthesis of Organoboron Reagents
A Synthesis f r k Organoliihiim or Magnesium
Reagents
B. Hydroboration of Alkenes and Alkynes
C. Haioboration of Teninal Alkynes
D. Miscellaneous Methods
Compounds and Their Mechanism
A. Cross-Cowlino Reaction
111. Palladium-Catalyzed Reactions of Organoboron
B. Other Catalyti; Process by Transition-Metal
Complexes
IV. Cross-Coupling Reaction
A. Coupling of 1 -Alkenylboron Derivatives:
Synthesis of Conjugated Dienes
6. Coupling of Arylboron Derivatives: Synthesis
of Biaryls
C. Coupling of Alkylboron Derivatives
D. Coupling with Triflates
E. Synthesis of Vinylic Sulfides
F. Coupling with lodoalkanes: Alkyl-Alkyl
CouDlino
G. Coupling with Other Organic Halides and
Boron Reagents
V. Head-to-Tail Coupling
VI. Carbonylative Coupling
VII. Alkoxycarbonylation and Dimerization
VIII. Conclusion
Attachment: Palladium-Catalyzed_Cross-Coupling_Reactions_of_Organoboron_Compounds_.pdf (3.7MB)
This file has been downloaded 810 times
Cloner - 1-11-2006 at 08:57
Another class of reactions that are used is organometallic coupling of arylbromides (or iodides). This can be done using a grignard approach or
organolithium, acting on another similar molecule (ie an aryl halide). In this reaction nickel catalysts are used instead of palladium.
xwonchem - 14-11-2006 at 02:52
how to link two naphthyl ?
using Ullman reaction
Ozone - 14-11-2006 at 20:42
Hello,
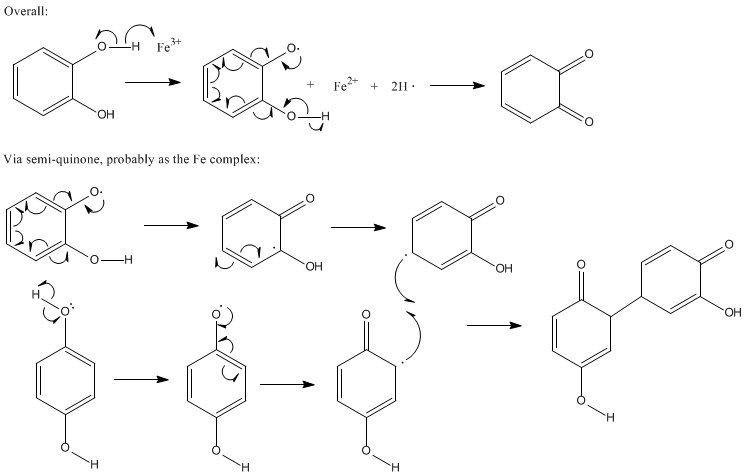
How about the oxidative coupling of phenolic rings? This can be done in water using Fe3+ and air. This works with o- or p-dihydroxybenzene moieties,
and the oxidation yields, overall, H2O2, which regenerates the Fe3+ catalyst.
I give the general idea, below. Oh yes, and the final "product' can do this over and over; it will actually present as the aromatized species, which
is favored by about 151 KJ/mol.
Cheers,
O3
[Edited on 15-11-2006 by Ozone]
xwonchem - 28-11-2006 at 23:43
Hi Ozone!
The last product of reaction can be used in next steps and I think that it is aromatized to form biphenyl. If I replace phenol by
stitutated-napthol, the reaction also likes that .Is it ok? However, how the way to aromatized final products?
Ozone - 29-11-2006 at 16:37
OK. Stitutated, what's that? Napthols should work provided that 1) there are two hydroxyls and 2) they are ortho or para (meta cannot provide a
suitable resonance hybrid). This is easily aromatized, the carbonyls will give up their electrons in a "tautomeric" way leading to the tetra hydroxy
substituted product. The driving force for this is quite large and hence, the aromatic (and conjugated!) product will be formed. Note, the regenerated
o-dihydroxy moiety can participate in the chemistry again and again. This can be controlled to some extent but limiting the availability of oxygen.
EDTA will hault this rxn by sequestering the catalyst.
Cheers,
O3
xwonchem - 30-11-2006 at 20:09
Ozone, Could you talk to me the conversion degree of this reaction is high or low?
On the other hand, I think that radical reaction is very difficult to control and it will happen many ways. How you think about that?



