3 )
Halogens will replace hydrogen attached to nitrogen
Here are current applications of Fluoro amines
http://www.pat2pdf.org/patents/pat6395899b1.pdf
http://www.pat2pdf.org/patents/pat6417355b1.pdf
Hexachloromelamine
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=1705...
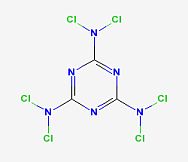
This appears to be an explosive given the dichloramine groups.














 Figure 1
Figure 1 Figure 2
Figure 2 Figure 3
Figure 3  Figure 4
Figure 4 