
woelen - 23-3-2016 at 23:52
For my element collection I also want to add small samples of colloidal gold. I know how to make this from HAuCl4 and Na-citrate, but the solutions
tend to be unstable on storage. Over time, the particles tend to aggregate into larger ones and settle at the bottom.
Maybe someone else over here has experience in making stable colloidal gold "solutions" which can be ampouled and stored indefinitely?
I have a 4% solution of HAuCl4 as starting material. I want the red variation and the blue/purple variation, the latter having IIRC a larger particle
size.
j_sum1 - 24-3-2016 at 00:38
Snap.
I have exactly the same idea for m collection.
I am also interested in something that lasts long term. I have heard of a very large number of agents used for colloidal gold but don't know much
about of any of them.
CuReUS - 24-3-2016 at 04:16
this might be useful
https://en.wikipedia.org/wiki/Gold_number
unionised - 24-3-2016 at 05:54
The colloidal gold that stained my kitchen counter-top was so stable that I ended up painting over it to cover the purple stain.
Sulaiman - 24-3-2016 at 06:46
I do not know anything about colloidal gold but I have made and stored colloidal silver
by simply passing a current through two silver electrodes in distilled water
The initial megohms of resistance drops VERY quickly as ions enter the water
More coulombs gives more suspended (and I believe ionic) silver in the water
Continued current starts to grow the size of the colloidal particles after about 20 ppm.
Caution: from memory, please verify.
Oscilllator - 24-3-2016 at 14:34
I'm sure you have already thought of this, but why not try using an emulsifier?
A quick google search seems to suggest there are lots of them readily available because they are used in food products, so it's worth a shot surely.
Sniffity - 25-3-2016 at 19:10
Hey,
Might not be exactly what you're looking for; but gold nanoparticles can be prepared using a certain polymer, chitosan, as a reducing and capping
agent. I've seen this experiment succesfully replicated at our polymers lab in my university. The red colour you wish to obtain was clearly visible.
I'm not sure about long term storage, but in chitosan does seem to be good at stabilization of a large number of compounds.
Here's the scientific paper we used as reference for this experiment:
http://www.sciencedirect.com/science/article/pii/S0167577X14...
I happen to have this paper in Pdf format, but I have no idea how to upload here. If anyone lets me know of a way to share it, I gladly will.
Hope this helps!
Tsjerk - 26-3-2016 at 01:14
Maybe coupling with bovine serum albumin (BSA) would work? This protein is really stable and is regularly used as coating for hydrophobic (e.g.
plastic) surfaces in order to prevent adhesion of hydrophobic dyes. I could imaging BSA will prevent particles to aggregate.
http://openwetware.org/images/c/c9/110417_SDP_TannicAcidAuNP...
In this method dialysis of the sample is described, but that would be unnecessary if it is just to prevent aggregation during storage I guess.
If you think this could be a solution I could sent you a milliliter of solution, as we get these tubes included with about every order we make.
[Edited on 26-3-2016 by Tsjerk]
fluorescence - 26-3-2016 at 04:28
I didnt even know it reacted. There was a good paper on the preparation of diferent sizes of collodial Gold since elemental gold nano particles can be
used for a visible light with TCPO, that is actually quite cool in the mechanism. So they tried different ways how to reduce the gold in different
sizes. Maybe changing to bigger particle size could improve the stability ?
http://pubs.acs.org/doi/abs/10.1021/ac050882q
Chalo - 21-8-2022 at 13:16
The most stable gold nps involve self-assembled-monolayers that coat the particle and keep them from sticking together as aggregates; even better if
the particles are charged and repel each other; and still better if they are in a nonpolar solvent like hexane that increases the repulsion. Particles
like that were a bear to make, taking days and exotic detergents and $$$, or you could buy them from sigma for a kilobuck per 5 mls. BUT!!! recently
someone published a ten minute inexpensive protocol. These could be ampouled indefinitely and are beautiful.
I have the pdf but dont know how to upload it.
See video:https://www.youtube.com/watch?v=nqkwM9o1s-w
see paper: Charged Gold Nanoparticles in Non-Polar Solvents: 10-min Synthesis and 2D Self-Assembly
Matthew N. Martin, James I. Basham,† Paul Chando, and Sang-Kee Eah*
clearly_not_atara - 24-8-2022 at 16:56
Stained glass 
Chalo - 26-8-2022 at 17:24
Figured out how to attach the paper here.
could not get this to work, NaBH4 reacts instantly but also aggregated instantly into tiny dark blue spheres.
Hope someone else tries this. If you need dodecanethiol, I have 100 mls, so given only a microliter needed per reaction I can spare enough for a few
thousand reactions.
The applications for stable AuNPs, especially as redox catalysts, are endless check literature.
Attachment: goldnano martin2010.pdf (2.1MB)
This file has been downloaded 299 times
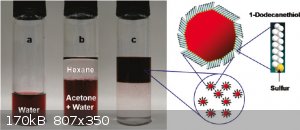
Sulaiman - 27-8-2022 at 01:27
I noticed that gold precipitated from chloroauric acid gives smaller particles at lower acid concentrations.
Try working with more (100x?) dilute solutions?
pneumatician - 8-11-2022 at 18:06
maybe a "tincture"/extraction of Gold with ether???
this guy use Rosemary oil = colloidal Au
https://vimeo.com/142743073
or...
https://www.youtube.com/watch?v=MDF2TXZIuj0
this precipitate/go rancid/ferment...??? I don't known till I make a test :-)
Junk_Enginerd - 17-11-2022 at 07:49
This makes me think of NileRed's video about preparing ferrofluid. That's also a case where the particles really want to aggregate, but are stopped
from doing so by coating the nanoparticles with soap molecules. It should be just as applicable to gold particles.
clearly_not_atara - 17-11-2022 at 21:21
Most emulsions need surfactants to stabilize them.
Lithium - 19-11-2022 at 19:37
Colloidal systems are inherently unstable. Particles dispersed in solution have a tendency to aggregate: think of paint, of milk, of muddy river
water. Colloids aggregate over a wide range of timescales: seconds, hours, weeks, years. But they all do. A variety of tweakable features have a role
to play: particle size, the composition of the continuous medium, the composition of the particle and the particle's surface. I will briefly discuss
them in turn.
Aggregation is driven by entropy, or thermodynamics, or both. When a bulk material is dispersed as finely divided particles in a continuous medium,
the interfacial area between those two mediums is made much much larger. A grain of gold divided into nanosized particles and dispersed into water has
a significantly larger area where the gold atoms are in contact with water molecules. Because 'like favours like', the gold atoms prefer contact with
other gold atoms and the water molecules prefer contact with other water molecules. As a consequence, it is thermodynamically favourable for the
system to reduce the area of contact between gold atoms and water molecules.
Increasing this area is rolling the ball up the hill. The ball tends toward the bottom.
Broadly speaking, nanoscale particles tend to aggregate less frequently. For example, clinically-used liposomal preparations are on the order of 200
nm in diameter or less. Particles of this size take longer to aggregate; greater biocompatibility and a longer circulation half-life is evidence of
this. The substantially reduced tendency toward aggregation is a unique feature of nanoscale materials which is partly responsible for the recent
explosion in their study. Size matters.
The composition of the continuous medium matters. Specifically, the species that are also present in the continuous medium. Ionic substances
substantially influence aggregation rates. In the case of NaCl, higher concentrations promote aggregation. This effect can be dramatic. Charged
species disrupt the electric double layer that naturally forms around a colloidal particle, causing a charge-screening effect that reduces the
repulsive forces between particles. This effect can also be seen with proteins and peptides that adhere to the particle's surface. Note that some
ionic species such as Li+ and F- have outlier-type effects on this layer.
The composition of the particle matters, but mainly because it decides what you can stick to the surface and how. Steric stabilisation is possibly one
of the most widely used or effective techniques to improve the stability and shelf-life of colloidal systems. Their mechanism is very simple: two
particles can't aggregate if they don't touch. There are two broad types: surfactants bound to the particle itself, and surfactants in the continuous
medium that naturally stick to the surface of the particle. The first is more reliable or robust; the second can be simpler or more practicable to
use. They are both very effective.
Aside:
The tried-and-true method for characterising particle size is dynamic-light scattering (DLS). It's rapid, simple, and cheap. I am certain that an
amateur could construct an instrument capable of carrying out this technique, if one has not already done so. This is a call to the amateur
experimentalist out there with a little more raw material and engineering savviness than most, but who still has some time to tinker. A simple and
cheap DLS instrument reproducible by the amateur opens one of three doors to the field of nanoscience. The other two include communicating safe
practices for experimentation and handling waste that are unique to nanomaterials, and a technique to gather information on the crystal structure of
the material. I'm sure both will take time, the second a lot more than the first.
I am a little rusty on the foundational theory, but there is a lot out there to read on colloid and interface science. Take care.
pneumatician - 20-11-2022 at 08:41
Well, the easy method is to tint some water with from yellow to deep purple and say to anybody: hey look, this is colloidal Gold! :-D
or go a bit more deep inside the "secrets" of colloidal metals...
http://www.silvermedicine.org/colloidal-silver-medical.html
https://www.elixa.com/colloidal-silver-a-closer-look/
https://healthwyze.org/reports/633-making-and-using-genuine-...
snake silver oil ha ha ha
https://www.quantumbalancing.com/news/cs_lunar.htm
or go to real Gold "spiritual" better than any colloidal shit, but this is for "quantum" minds only...
[Mode end: pedantic]
--> error no pedantic Mode on
[Mode end: genius]
Ok_
Lionel Spanner - 21-11-2022 at 14:00
In my experience experience, 0.1% low-acyl gellan gum gelled with calcium ions does an excellent job of suspending solids in water and minimising
aggregation, while adding little if any viscosity. Never tried using it with colloidal gold or silver though.

