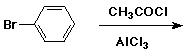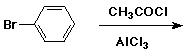Colli - 9-4-2006 at 14:38
Please help...?
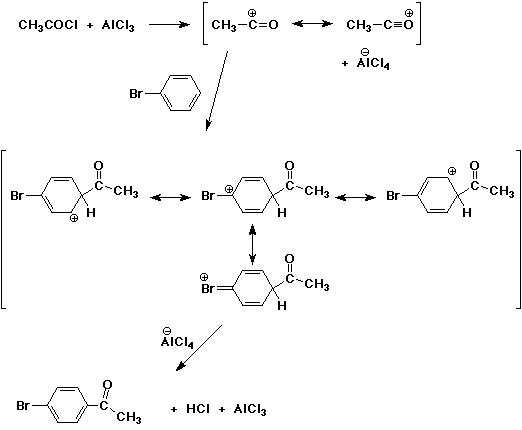
I'm doing the mechanism of reaction for the friedel-crafts acylation of bromobenzene, using acetic anhydride, aluminium chloirde and
dichloromethane... I've washed the organic product with dichloromethane, water, 2m naoh, and half saturated solution of nacl.
I just can't figure out what other products can be formed from this reaction other than 4-bromoacetophenone...? Bromine is ortho, para directing and
the acetyl group is meta directing and strongly deactivating, right? So, how could any other product be favoured?


[Edited on 9-4-2006 by Colli]
praseodym - 9-4-2006 at 21:10
I think this may be able to help you: