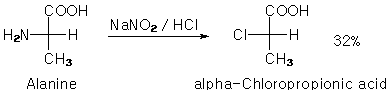Originally posted by IPN
100ml of 80% lactic acid was refluxed for 2h in an oil bath with 85ml 35% HCl and 1mol of ZnCl2. Mixture was initially golden yellow and clear but
turned dark while refluxing. Some gas was evolved for a short period. I guess it was just HCl. Should I now distill the mixture? I don't have a
vacuum source but CRC doesn't mention the 2-chloropropionic acid decomposing at its boiling point (185C). Everything else should come over before
the chloro-acid starts to distill (lactic acid decomposes in atmospheric distillation, but into what?). |

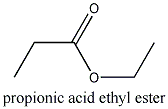
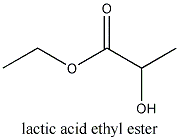
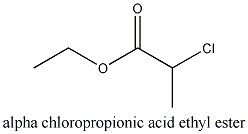
 )
)

 even though I carefully turned the s.funnel up and down taking care not
to shake it. So now I guess I just have to wait for it to separate.
even though I carefully turned the s.funnel up and down taking care not
to shake it. So now I guess I just have to wait for it to separate.

 Here is what he had to say about it:
Here is what he had to say about it: