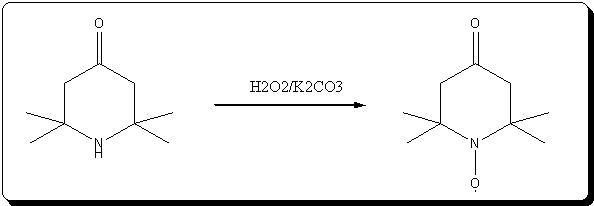
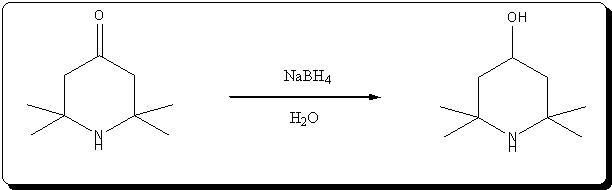
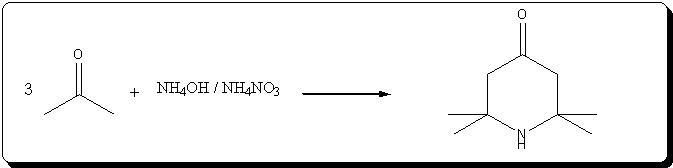
A. 2,2,6,6-Tetraperdeuteromethyl-4-oxo-3,5tetradeuteropiperidine (Triacetoneamine-d16) (V)
A mixture of ammonium-d4 chloride (3.45 g, 0.06 mole), acetone-d6 (99.5 atom %D, 12.5 ml, 0.15 mole), anhydrous sodium carbonate (3.18 g, 0.03 mole)
and magnesium oxide (3.0 g) was added to a 250 ml round-bottomed flask. The flask was capped with a rubber septum and wired, then the reaction mixture
heated in an oil-bath at 50° C. for 3 days. After cooling, 20 ml of acetone was added to the reaction mixture and the resulting mixture was filtered.
The recovered solid was crushed into powder, washed with 15 ml of acetone and then filtered with suction filtration. The combined filtrates were
concentrated to dryness. The resulting red liquid (7.2516 g) was distilled under reduced pressure to obtain 4.7480 g (56.7%) of a bright yellow liquid
(b.p. (boiling point) 54°-55° C./1.9 mm Hg) that solidified when chilled in a dry ice/acetone bath. The solid product subsequently was used without
further purification. Recrystallization of an analytical sample from anhydrous diethyl ether yielded white crystals, mp. 57°-58° C. [lit. 58° C.];
IR(KBr, cm-1):3580(m), 3260(m), 2220(m), 1700(s), 1530(w), 1265(s), 1140(m), 1050(m), 930(w); 13 C-NMR (CDCl3): 31.03(m), 53.50(m), 54.88(s), 211.19
(s).
|




 I since procurred the stopper from my local hardware store!
I since procurred the stopper from my local hardware store! and I
added the 100 ml acetone needed.
and I
added the 100 ml acetone needed.
 ) to put the
distn setups up, and i was off to isolate my 2,2,6,6-tetramethylpiperidin-4-one.
) to put the
distn setups up, and i was off to isolate my 2,2,6,6-tetramethylpiperidin-4-one.



















 The
4-oxo-TEMPO qsynthesis can take a weekend's work at most if using NH3 gas, or a afternoon if mixing aq ammonia and acetone and forgetting it for a few
weeks, counting the distn and oxidation. I means, how much more available can it get? Ammonia and acetone
The
4-oxo-TEMPO qsynthesis can take a weekend's work at most if using NH3 gas, or a afternoon if mixing aq ammonia and acetone and forgetting it for a few
weeks, counting the distn and oxidation. I means, how much more available can it get? Ammonia and acetone  !
!

 ).
).


 ). In the
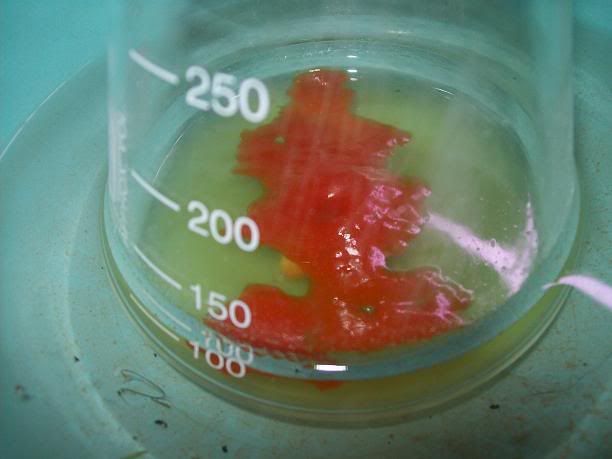
end I got a minute yield of a beige salt, with a small amount of black tar. Unfortunately the 'PYREX' (supposedly) baking dish decided to emit a wee
crack and then turn into shrapnel. I collected as much as I could, then started extracting that with 95(ish)% EtOH - the black tar is not seeming to
come through the large funnel, while the salt is dissolving and turning the resultant solution yellow as I write. I'll pour the filtrate back over the
shattered glass a couple more times and then discard the glass. I'll then remove the EtOH and see how I go (I'll simply reduce it as much as I can via
distillation, then crystallise).
). In the
end I got a minute yield of a beige salt, with a small amount of black tar. Unfortunately the 'PYREX' (supposedly) baking dish decided to emit a wee
crack and then turn into shrapnel. I collected as much as I could, then started extracting that with 95(ish)% EtOH - the black tar is not seeming to
come through the large funnel, while the salt is dissolving and turning the resultant solution yellow as I write. I'll pour the filtrate back over the
shattered glass a couple more times and then discard the glass. I'll then remove the EtOH and see how I go (I'll simply reduce it as much as I can via
distillation, then crystallise).













 . I think a little less acetone could be added in the second step, I followed the
second series of examples in the patent, but the first one used 1/3 the amount of acetone in the second step.
. I think a little less acetone could be added in the second step, I followed the
second series of examples in the patent, but the first one used 1/3 the amount of acetone in the second step. :
:
 ) and presenting a remarkable workup for the
H2O2/CO3 2- (which is much cleaner than the WO4 2-! I wonder why they even want to use versanate and tungsate salts when carbonate works better?! ),
that I am attaching because it's so usefull.
) and presenting a remarkable workup for the
H2O2/CO3 2- (which is much cleaner than the WO4 2-! I wonder why they even want to use versanate and tungsate salts when carbonate works better?! ),
that I am attaching because it's so usefull.













































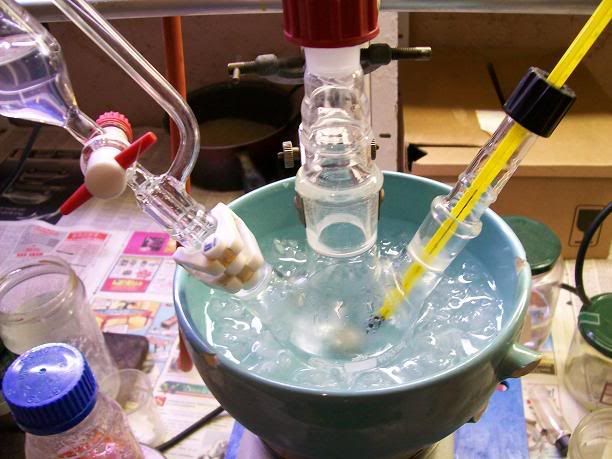

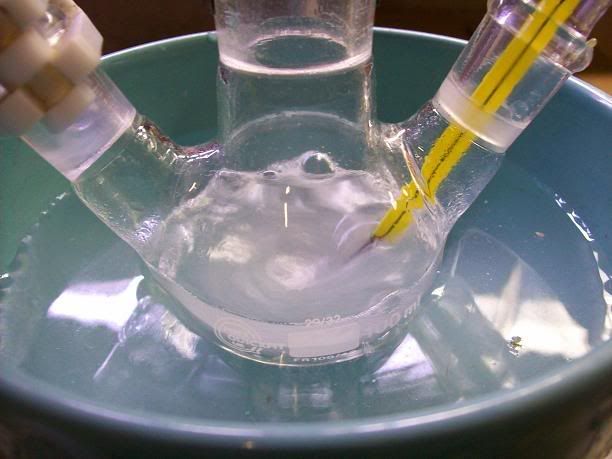

 ).
).













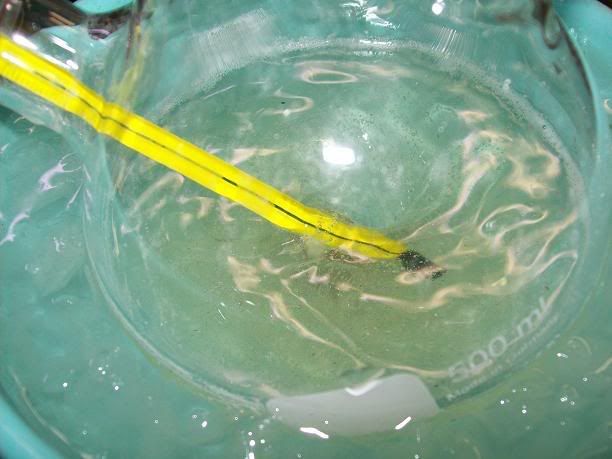

















 ) that it can be removed with aid of dioxane. I just
added 4x20 ml of dioxane with stirring and careful pipetted off liquid after each portion of dioxane.
) that it can be removed with aid of dioxane. I just
added 4x20 ml of dioxane with stirring and careful pipetted off liquid after each portion of dioxane. .
.


 1.23 (s, 12,
CH<sub>3</sub>
1.23 (s, 12,
CH<sub>3</sub> , 2.26 (s, 4 H, CH<sub>2</sub>
, 2.26 (s, 4 H, CH<sub>2</sub>


