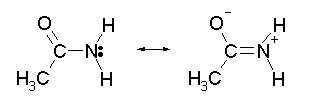CH5CI.N205,; mw 160.52;
N 17.46%; OB to C02 +4.98%; v hydroscopic and unstable white crysts; mp, explds on heating without burning. V sol in w. CA Registry No [1872-07-6].
Prepn is by the interaction of equimolar amounts of urea and perchloric acid in acetic acid followed by solvent evapn. Its
impact sensy is comparable to that of TNT
(Refs 1 and 17a) The perchlorate has been incorporated into
more than several expl compns. Shiino et al claim in their patent (Ref 23) that a liq expl of high deton vel can be made by dissolving solid aromatic
nitro compds into aq urea perchlorate.
Thus, PA (5) is dissolved in 85% aq urea perchlorate (95 wt p) to obtain a liq expl having a d of 1.6g/cc and a deton vel of 6520rn/sec. In the
invention of Fujiwara et al (Ref 24) an expl slurry of improved stability is claimed consisting of a mixt of combustible solids in aq urea perchlorate
soln. Typically, powdered K perchlorate
(30) and powdered ferrosilicon (30) are
added to 85% aq urea perchlorate (50 wt p) to form a slurry-like expl having a d of 1.7 lg/cc and a deton vel of 3700m/see, as well as high stability.
In another patent, Shiino et al (Ref 25) claim a process for the prepn of the expl
perchlorate, per se. Thus, 430g of urea are added slowly to 1000gof715Z0 aq perchloric acid at <20° to give the 1:1 urea perchlorate salt crysts.
The patent shown in Ref 26 suggests the use of the perchlorate in an underwater blasting slurry when incorporated with materials
such as PA. The claimed impact sensy is > 60cm (5-kg wt from 60cm resulting in 0/6 trials).
Fujiwara et al, as a result of their work, (Refs 29& 30) with aq urea perchlorate solns mixed with various organic substances, report that with
detonatable materials such as PA, the solns
formed are impact insensitive but powerful
expls exhibiting two distinct deton modes: low vel deton (LVD) (’u 1900rn/see) and high vel deton p 6000m/see). Mixts with nondetonatable
substances as ~trobenzene or dimethylformamide show only LVD, requiring an extremely high critical initiation pressure of approx 40kbar.
|