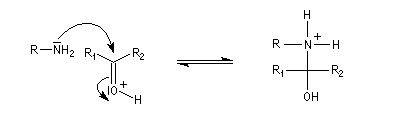Bloodshine - 26-12-2004 at 15:40
Hi,
A friend of mine had an idea for an OTC synthesis of Guanidine.
Here is his idea:
The synthesis of an imine is made by the adition of a primary amine on a carbonylated compound which gives a non-stable amino-alcool
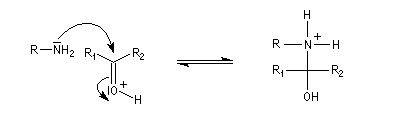
And then the deshydration of this amino-alcool in an acidic environment gives the imine
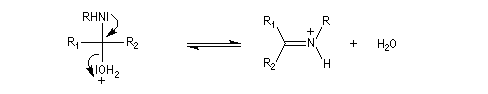
So he was wondering if it could be possible to synthesis Guanidine

by the action of NH4OH on urea (NH2)2C=O?
It would be an easy synthesis of Guanidine and then to some others energetic materials like guanidine nitrate, guanidine carbonate, nitroguanidine...

[Edited on 26-12-2004 by Bloodshine]
kyanite - 26-12-2004 at 16:35
I'd say that'd probably work. You might want to use ammonium chloride instead of ammonia water. That way you won't be watering down
your reaction. Also it could at the same time be the weak acid needed to finish the reaction.
The only thing I'd might be worried about is the stability of the imine. I think I read somewhere that primary imines aren't as stable as
secondary/tertiary.
Good luck!
sylla - 27-12-2004 at 00:24
the preparation of guanidine salts has already been discussed here : http://www.sciencemadness.org/talk/viewthread.php?tid=2762
US #4,535,185 may help you