
eidolonicaurum - 4-4-2014 at 11:38
I have had a good look on the internet for hypothetical biochemistry for silicon-based lifeforms, but have come up with a total blank. My general
theory is that these organisms respire with chlorine, and things like water and oxygen are as toxic to them as chlorine is to carbon-based organisms.
The theory is that silicon tetrachloride is produced, along with hydrogen chloride and sulphur dichloride. (Sulphur replaces oxygen). Obviously, the
temperature these organims need is well above ~60C, probably closer to 100C. I have come up with theoretical silicon amino acids, carbohydrates, and
lipids. By biochemistry knowledge is A-level, so I appeal to the discussion board's collective intellect and knowledge to spot my (probably obvious)
errors. One problem I have not solved is which solvent could I use for these organisms, since it has to be liquid at at least 100C, and cannot contain
oxygen. In carbon based organisms, water is the solvent.
Something I am fully aware of is that silicon chains longer than 4 silicons are incredibly difficult to synthesis, and the longest ever observed are
~8 silicons. Methods of getting around this would be much appreciated.
The pictures uploaded are: Generic amino acid, an example lipid, ATP, nucleo tides
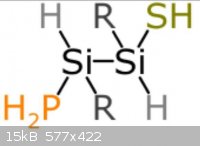
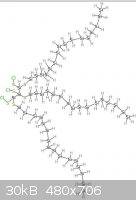
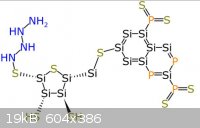
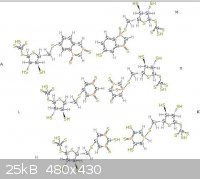
Steam - 4-4-2014 at 12:05
Polyscilicates generally have alternating silicon oxygen bonds.
gregxy - 4-4-2014 at 12:31
There isn't much on the internet on silicon based life,
I guess it was just a Star-Trek thing.
However one article points out that Si does not form double bonds with other Si atoms or S or P (or at least not very strong ones).
Steam - 4-4-2014 at 14:00
However there are some sulfur reducing bacteria out there which can use sulfur instead of oxygen. Instead of making H2O they make H2S.
This however is an interesting subject because Si can form complex molecules with germanium and Tellurium.
Si based life is very unlikely, but then again, when one looks at the complexity of our carbon based lives, it may not seem so far-fetched!
