
Oxydro - 7-11-2004 at 07:15
I was just thinking, for no really good reason, about the hydrogen+chlorine reaction as described in "The ... way of making HCl!" especially the talk of balloons full of the mix.
I started wondering, what would happen if you had such a balloon in a dark environment, or red light would work I guess, and you then used a camera
with flash to take a picture of it. The intense light of the flash would likely initiate the reaction, and maybe you could get a good picture of it in
the process.
The problem I anticipate is timing. I fear that the interval between the flash and the end of the exposure would be too short for the reaction to
develop.
I think I'll try it as soon as I can get access to a good (digital) camera... thoughts anyone?
Esplosivo - 7-11-2004 at 07:34
Chlorine would probably react with the plastic of which the balloon, although I don't know exactly what would happen in the dark. Probably the
reaction with the plastic is a free-radical reaction which would not occur in the dark, but you better check it out rather than having a chlorine
balloon 'explode' in you face.
And as you said timing is a major problem. Such photos are usually taken with cameras having an enormous capture rate of hundreds of frames per
second.
And just a curiosity but how are you thinking of filling up a balloon with such gases? Electrolysis of NaCl solution would not work, since pressure is
needed to fill up the balloon - which cannot be created by simple elctrolysis unless it is being carried out in a sealed container.
Oxydro - 7-11-2004 at 07:54
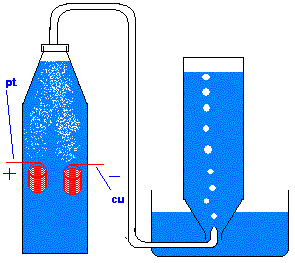
The setup I envision is similar to this (hopefully attached), but running conc. NaCl solution instead of NaOH, H2SO4, whatever, so that Cl is
generated as opposed to oxygen. also obviously instead of the tube leading to the bottle in the pneumatic trough there would be a balloon over the
mouth of the bottle. I would first test the electrode seals by inflating a balloon in the conventional (oral - air) way and attaching it to the
bottleneck -- better air leak than H2/Cl2 mix.
hodges - 7-11-2004 at 08:42
I would think that the balloon would block the UV light from the flash.
I have seen notes on a chemistry lecture where the professor demonstrates H2 + Cl2 to the class. He mixes the gases in a tube and then tells the
class he wants to take a picture of the results. When he snaps the picture, BOOM! Not sure if this gets captured on the film, though.
Oxydro - 7-11-2004 at 09:36
I'm taking the idea from this from Bromic's post on his teacher demoing the H2+Cl2 reaction to his class in a balloon, in the thread I
linked to in the first post in this topic. I suppose it doesn't have to be a balloon, actually a PE (or whatever it is?) pop bottle would
probably be better... the only reason I suggested a balloon is the lack of shrapnel.
I have experimented in the past with midsized hydrogen/oxygen gas mixes and I have found that they can have quite the effect, and hydrogen/chlorine
is a fair bit more energetic I think. See the results from a modest 4-liter bottle of oxy/hydrogen mix, electrically initiated inside a sturdy
cardboard box, in the attachment -- therefore I am not too keen on getting near H2+Cl2 in a hard container.
I'm not sure whether I'm happy or disappointed that the idea of a camera flash to ignite it has been used before! 
 At least I know it
can work!
At least I know it
can work!
[Edited on 7-11-2004 by Oxydro]

vulture - 7-11-2004 at 13:06
If you can, find a camera that'll allow you to use flash slave mode. This means the camera will wait for an external flash to go off before
triggering it's own flash and taking the picture. This could be just the right bit of time you need.
If not, ignite the mixture electrically and let the flash of the explosion activate the camera.
You'll need a camera with a high flash synchronisation speed though. Most consumer cameras only go to 1/60 of a second max. I know that Nikons
D70 has a flash sync of 1/500 but that camera is quite pricey and I doubt you are going to rent it for this kind of experiment.
Tacho - 8-11-2004 at 07:41
Instead of a baloon, think of using a ziplock bag like here. I sugest you produce the gases in plastic bags too. Take a look at that site.
Good luck.
[Edited on 8-11-2004 by Tacho]
[Edited on 8-11-2004 by Tacho]
garage chemist - 8-11-2004 at 12:53
Oxydro, your gas generator won't work!
It will generate nearly pure hydrogen only, with a tiny chlorine contamination, way too little to render the mix explosive.
At the cathode, hydrogen AND NaOH is generated. The NaOH then reacts with the chlorine at the anode (assumed the chlorine doesn't eat the
electrode first- use either graphite or platinum, no other metal will work!) and forms NaOCl and NaClO3.
You have to electrolyze HCl instead. This will yield a perfectly stochiometric Cl2/H2 mix.
Oxydro - 8-11-2004 at 19:00
It works. I have generated chlorine & hydrogen with it before. The current density at the anode (which is some coiled up Pt wire, btw) is high
enough that quite little of the chlorine gas is absorbed. But, since I have it, I will take your advice and use HCl.
I have also run it at very low current densities, so that nearly all the chlorine is absorbed. I bleached dark blue & red cloths white with the
resulting sodium hypochlorite solution.
Actually, I just built a nice (well, not really nice) new gas generator. Picture attached. The gas is lead off through tubes glued through the caps
[not shown], which also hold the electrodes (one the Pt wire coil, one a copper coil. Yes, it's made of an ice cream container. A permeable
membrane can be inserted to act as a salt bridge -- actually this is just a concept so far. Will cardboard help prevent the mixing of the electrolytes
effectively? Just to save my chlorine from the hydroxide 
Tacho - nice links... I am now a born again gas bag chemist  . Actually, I have
used bags for gases before but I was too lazy to seal them right so I started just using pop bottles inverted in a tub as a sort of pneumatic trough.
Works surprisingly well, at least for anything that doesn't dissolve in water! I've always liked working with gasses for some reason
. Actually, I have
used bags for gases before but I was too lazy to seal them right so I started just using pop bottles inverted in a tub as a sort of pneumatic trough.
Works surprisingly well, at least for anything that doesn't dissolve in water! I've always liked working with gasses for some reason
Vulture -- No, no expensive cameras for me  . I'm just going to try assorted
crappy cameras (plus a decent digital) at different settings until I get lucky (not that I'll know immediately, except with the digital).
. I'm just going to try assorted
crappy cameras (plus a decent digital) at different settings until I get lucky (not that I'll know immediately, except with the digital).
Since I share an basement apartment with 3 other people, and the landlord lives upstairs, it's rather hard to get any chemistry time. The one
room that they wouldn't mind me using is completely enclosed, so I can't do *anything* that involves fumes most of the time. I guess
I'll need to wait until the next time I visit my parent's place to try this... they've got a few acres of field as well as a couple of
small buildings I can use  ... plus Mom owns the digital camera.
... plus Mom owns the digital camera.
[Edited on 9-11-2004 by Oxydro]

garage chemist - 13-11-2004 at 04:48
Heres a video of a test tube full of H2/Cl2 mix being ignited by a camera flash (neither the flash nor the explosion are on the video, because they
happen between two frames):
www.seilnacht.com/film/chlork.mpg


 At least I know it
can work!
At least I know it
can work!
 . Actually, I have
used bags for gases before but I was too lazy to seal them right so I started just using pop bottles inverted in a tub as a sort of pneumatic trough.
Works surprisingly well, at least for anything that doesn't dissolve in water! I've always liked working with gasses for some reason
. Actually, I have
used bags for gases before but I was too lazy to seal them right so I started just using pop bottles inverted in a tub as a sort of pneumatic trough.
Works surprisingly well, at least for anything that doesn't dissolve in water! I've always liked working with gasses for some reason
 . I'm just going to try assorted
crappy cameras (plus a decent digital) at different settings until I get lucky (not that I'll know immediately, except with the digital).
. I'm just going to try assorted
crappy cameras (plus a decent digital) at different settings until I get lucky (not that I'll know immediately, except with the digital).  ... plus Mom owns the digital camera.
... plus Mom owns the digital camera.