And finally, Axt, did your propyne smell terrible, mine did
 , I was expecting it to
smell something like propane which I find has a semi-pleasant smell.
, I was expecting it to
smell something like propane which I find has a semi-pleasant smell. 
| Quote: |
| Quote: |
| Quote: |


| Quote: |
| Quote: |
 , but that is probally
because I am in a province with a lot of petrochemical resources/industries. I also managed to get around paying for a 50$ adaptor by just using the
cheap burner head from my propane torch, unscrewing the flame adjuster off and attaching tubing to what was left of the burner assembly.
, but that is probally
because I am in a province with a lot of petrochemical resources/industries. I also managed to get around paying for a 50$ adaptor by just using the
cheap burner head from my propane torch, unscrewing the flame adjuster off and attaching tubing to what was left of the burner assembly. , I was expecting it to
smell something like propane which I find has a semi-pleasant smell.
, I was expecting it to
smell something like propane which I find has a semi-pleasant smell.| Quote: |
| Quote: |
 the soot makes the flames very bright!
the soot makes the flames very bright! | Quote: |


 . Sounds like yours is a different formulation to me, I dont know what MPS gas is.
. Sounds like yours is a different formulation to me, I dont know what MPS gas is. )
)| Quote: |
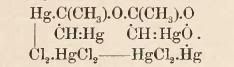
| Quote: |

