
chemrox - 1-2-2013 at 19:03
I am making a research chemical. It will be quite toxic and not at all suitable for consumption. The reaction starts with 100 mmol of
2-chloro-3-nitro-aniline, 100 mmol of N-phenethyl-4-piperidone and a few crystals of p-toluenesulfonic acid as a catalyst. The solvent is toluene
(tech grade). Apparatus consists of heating mantle, 3-neck, 1000 ml RB, Dean-Stark apparatus and 300 mm condenser. Additional apparatus is heat and
water flow control. I love the automation I found used at reasonable cost after three years of less than patient waiting. All the glass below the
condenser is foil covered. After 1 3/4 hours 0.3 ml water collected in the D/S. I will report the whole thing with a few photos after the
theoretical amt of water if collected or no more is developed over a 12 hour period. I'm thinking 4 days but it could go faster. I'm not sure enough
solvent was used for the 4-chloro. The boiling solution is still a bit cloudy. I may add 150-200 more. I have room for it. I used 375 for the
4-chloro and 225 for the NPP. So combined I have ~ 600 ml toluene in a 1000 rb. I could go to 7-800. Tomorrow I'll check and possibly add. Right
now I'm boiling and getting rid of reaction water. For those that like to know such things; the pot temperature is 113-4*C, the condensation level
is about 20% on the condenser. NB: this NOT a drug for human consumption. Nitro fentanyl analogues are stronger but so toxic they have no medical
use (I forgot where I read it, the paper is around here somewhere.) If you want to repeat this procedure be advised that you would have to make the
NPP. You cannot simply alkylate 4-piperidone as has been stated in a widely re-published "dry lab" by someone calling himself "French chemist." It
might work if 4-piperidone were just that. Next post will have the starting setup photo.
update: after 24 hrs 94.4 % (1.7 ml) water collected. Incredible! This is the fastest anilination of a piperidone I've seen in a few years of
experimenting with the reaction.
[Edited on 2-2-2013 by chemrox]
zed - 6-3-2013 at 19:31
Interesting note. A while back, since so many people have whined about difficulty obtaining NaBH4, I looked into producing it via ball-milling
anhydrous borates, with metal hydrides. Supposed to work pretty well with MgH2. Sadly MgH2 is expensive and hard to handle. On the other hand,
CaH2 can sometime be had very cheaply and might do the trick. I ran out of research "steam' and haven't followed up on it.
So, what does this have to do with your topic? Well, some of the references on "Ball-Milling" and NaBH4...Referred to reducing substrates...without
solvents, simply by ball-milling 'em with NaBH4.
Seems to me, one or two references suggested Fentanyl had been produced by such a treatment.
How are you going to execute your reduction?
psychronizer - 21-3-2013 at 05:32
Um...what do you mean when you mention "4 chloro"? I dont see how you can get 4 chloro anything from what your doing there? is that a typo? or am I
missing something vital here?, by the way...have you considered the possibility of reducing your imine that you've made with just sodium/ethanol? you
can make the sodium ead easy from igniting a mix of sodium hydroxide and magnesium powder (I think aluminium powder works too)....
chemrox - 28-3-2013 at 21:16
I promised to get back with photos but after rooting my android phone I still have to pull the backups of nandroid. First the synth by refluxing in
toluene led to a mess and distilling it at 6 mtorr made a black tar mess. The synth was repeated in ether with 4 a sieves and was quenched after 2
days. The yield was about 30% N-(4-chloro-3-nitrophenyl)-1-(2- phenylethyl)piperidin-4-amine. @psychro: figure it out. I've attached a diagram. I
will get back shortly with pics now that this beastly flu is over. Given the unexciting yield I doubt I will try to optimize this one.
Chromatography would be a better purification process. I would like to try this with m-toluidine. O-toluidine gave good yields and took about 2 1/2
days. Is NaBH4 hard to get? I shouldn't have sold 200 grams to a member.
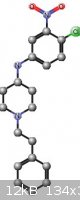
[Edited on 29-3-2013 by chemrox]
zed - 31-3-2013 at 12:03
NaBH4 is like sex. Some folks seem to be able to get it easily, while others have a very difficult time.
zed - 9-4-2013 at 16:55
Simple hydrogenation without the imine step, might work to produce a Fentanyl end-product, as hydrogenation reduces ketones slowly and imines
rapidly. Excepting of course, that in this case, the Nitro-group might also be reduced, and in general.....hydrogenation may not produce the correct
Fentanyl isomer.
Is it so difficult to produce the imine? I've never worked with your materials, but I was able to produce the structurally similar Phenylhydrazone
of Cyclohexanone, by the simple expedient of mixing Phenylhydrazine and Cyclohexanone, in anhydrous ethanol, and driving off the solvent. Ethanol
loves H20, and it takes the water with it when it leaves. Might even be able to perform the operation under reduced pressure. Thereby keeping
temperatures down, and by-products to a minimum.
zed - 13-4-2013 at 13:15
Well, apparently, Pt/H2 can be used for this reduction. Someone in the past, misinformed me.
http://www.dtic.mil/dtic/tr/fulltext/u2/a250611.pdf
The military has shown considerable interest in synthetic opiates......Some of the most toxic materials in existence. The US ARMY owned the patent
for M99 for a while. These types of materials are less fussy to manufacture and store, than items like Sarin, and the argument can be made that they
are not Chemical Weapons, but medicines... (Though they could be very effective as chemical weapons, if you decided to use them that way...)
[Edited on 13-4-2013 by zed]
zed - 16-4-2013 at 12:34
There is also the possibility a one-pot type synthesis, utilizing the aniline, the ketone, and Sodium Cyanoborohydride.
Like Catalytic Hydrogenation, Sodium Cyanoborohydride reduces Ketones very slowly, and Imines very quickly.
http://organic.wsu.edu/files/348/Lectures/lecture%207-8.pdf
