And, must nickel rods be used? I can't get nickel..


 so i tried some simple
electrolysis. Using carbon electrodes, as close together as possible i first tested it in a salt water solution. It bubbled rapidly, and i plan to
attempt to make and capture the Hydrogen and fill a balloon with it. I then proceeded to try KOH. I heated it on my hotplate and put the electrodes
in. Soon it began bubbling rapidly, and forming a kinda grayish mix. However, i stoped that experiment. Yesterday i tried to heat some NaOH, but my
hotplate wasnt getting hot enough, so i put a torch to the crucible and it began to bubble away. This happened for about 30 min, while the NaOH was
turning a silvery black color. ( I suspect this was the C coming off, and they were a bit smaller than before). However, my crucible got a hole in it,
so i stoped. That sat overnight, and i then wetted the mix, to find some whitish specks on the side got hot when water touched it. I think if any Na
or K formed, the water from the + electrode converted it back to OH as soon as it formed. Today i made a crucible with fire cement and put an C rod in
the center. Hopefully that will prevent anything from getting to the material.
so i tried some simple
electrolysis. Using carbon electrodes, as close together as possible i first tested it in a salt water solution. It bubbled rapidly, and i plan to
attempt to make and capture the Hydrogen and fill a balloon with it. I then proceeded to try KOH. I heated it on my hotplate and put the electrodes
in. Soon it began bubbling rapidly, and forming a kinda grayish mix. However, i stoped that experiment. Yesterday i tried to heat some NaOH, but my
hotplate wasnt getting hot enough, so i put a torch to the crucible and it began to bubble away. This happened for about 30 min, while the NaOH was
turning a silvery black color. ( I suspect this was the C coming off, and they were a bit smaller than before). However, my crucible got a hole in it,
so i stoped. That sat overnight, and i then wetted the mix, to find some whitish specks on the side got hot when water touched it. I think if any Na
or K formed, the water from the + electrode converted it back to OH as soon as it formed. Today i made a crucible with fire cement and put an C rod in
the center. Hopefully that will prevent anything from getting to the material. | Quote: |
 you will recieve about 5 grams of Na.
you will recieve about 5 grams of Na. 

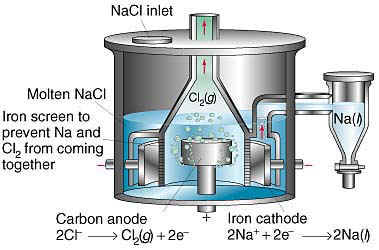

 When I see that a thread has new posts, I usually
don't reread the entire thread.
When I see that a thread has new posts, I usually
don't reread the entire thread.
 .
. ). The same problem with NaOH.
). The same problem with NaOH. 




| Quote: |

| Quote: |

 I found a nice picture of a Castner cell in a book at my library and made a photocopy, it details all the parts in a seperate paragraph
nicely although I liked the origional one on the first page better. I think that I might post a the picture of this one later though.
I found a nice picture of a Castner cell in a book at my library and made a photocopy, it details all the parts in a seperate paragraph
nicely although I liked the origional one on the first page better. I think that I might post a the picture of this one later though.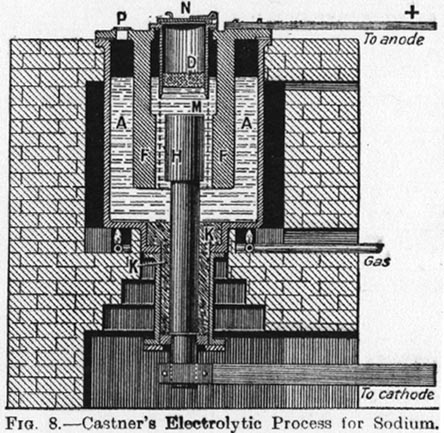
| Quote: |
| Quote: |
| Quote: |



| Quote: |
| Quote: |


 Sweet
Sweet 


 ), or that there is an oxygen deficiency in your local atmosphere (you wouldnt be writing this otherwise
), or that there is an oxygen deficiency in your local atmosphere (you wouldnt be writing this otherwise  )
) . I used the concave bottom of a pepsi can as the crucible and melted the
sodium hydroxide over an alcohol burner. Nickel electrodes were placed in the melt and 12V @ 2A from a car battery charger powered the electrolysis.
No sodium was evident for about a minuit but there was constant orange sparks. At around the minuit mark there was a crack which sent molten sodium
hydroxide flying, and now a tiny globule of sodium was visible. Not grey, but like mecury, nice and shiny. Most of the sodium hydroxide had
solidified by this time except for the 1 square centimeter between the 2 electrodes. After a while the sodium that was formed must have shorted the
electrodes because there was another crack, more molten material flying, and then the electrolysis stopped. I did 2 trials and both times I got loud
"cracks" when the sodium started and completed being formed. Strange.
. I used the concave bottom of a pepsi can as the crucible and melted the
sodium hydroxide over an alcohol burner. Nickel electrodes were placed in the melt and 12V @ 2A from a car battery charger powered the electrolysis.
No sodium was evident for about a minuit but there was constant orange sparks. At around the minuit mark there was a crack which sent molten sodium
hydroxide flying, and now a tiny globule of sodium was visible. Not grey, but like mecury, nice and shiny. Most of the sodium hydroxide had
solidified by this time except for the 1 square centimeter between the 2 electrodes. After a while the sodium that was formed must have shorted the
electrodes because there was another crack, more molten material flying, and then the electrolysis stopped. I did 2 trials and both times I got loud
"cracks" when the sodium started and completed being formed. Strange. 
| Quote: |
| Quote: |
| Quote: |
| Quote: |
| Quote: |