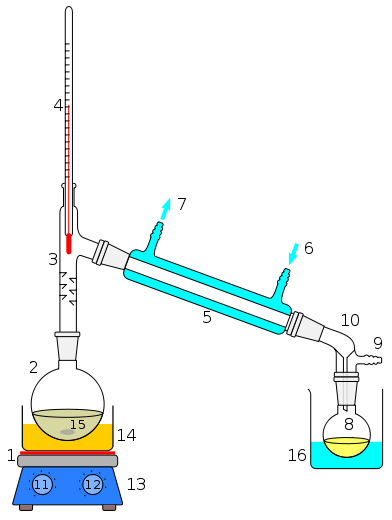
No, the outside temperature of the 3-way adapter and the BP of the liquid at 170*C suggests that the liquid will reflux and drip back into the flask WAY before going anywhere near the condenser.
Is it feasible to do the distillation indoors where there is no wind? Add some fibreglass wool insulation between the layers of foil if possible and if necessary heat the outside gently with a heat gun or hairdryer. Turn off the magnetic stirrer for about 10 seconds... .when the vortex has disappeared, can you see bubbles forming and rising to the top? This will probably tell you if it's boiling or not. Be certain when measuring with the infrared gun to hit the temperature of the liquid, not the hotplate itself. Can you see any other signs of actual boiling? Can you see little drops of liquid entering the flask again from the bottom of the fractionating column?
Also, how fast is the water entering the aspirator? A low speed (low pressure) will produce a very weak vacuum . . .perhaps your mains water pressure was different on that day where it worked.
Is this, or something similar, what you have for supplying the vacuum?

Nope what you see in the picture is the so called aspirator aka Pond Pump attached to an adapter which is attached to the water hose inlet which is attached to the condenser when I turn on the pump it fills really quick like within maybe 20 seconds? I will go back down now to get better pictures my lab top is not ideal for this lol
[Edited on 3-6-2012 by Hexavalent]