Lithium Electrowinning
Lithium is prepared by the electrolysis of fused lithium chloride in a lithium chloride - potassium chloride bath at 400 to 450C held in a cell
designed somewhat like a Dow magnesium cell or the Downs cell for sodium production so that the intermixing of the lithium metal and the chloride gas
is prevented (See Magnesium Electrowinning and Sodium, Electrolyte Production.) The LiCl-KCl mixture is approximately the eutectic mixture (about 45
per cent LiCl) whose melting point is 352C; the melting point of LiCl is 606C. Compared with the use of fused LiCl alone, this mixture improves
optimal efficiency, decreases corrosion problems, minimizes deteriation of the graphite anodes, and permits continuous operation.
The raw material is lithium chloride of high purity, particularly with respect to water and sodium content, although water can be removed to a degree
from the bath by pre-electrolysis at low amperage. Since lithium chloride is very hygroscopic, the handling of it to deter water pickup is a problem.
The physical properties of lithium and the LiCl-KCl eutectic mixture influence the cell design. Lithium melts at 179C and at 400C has a density of
0.49 g/cc, a viscosity of 0.402 centipoises, a vapor pressure much less then 1 mm Hg, and a surface tension of about 400 dynes/cm. The eutectic
mixture has a density of 1.65 g/cc at 450C and a viscosity of 5 centipoises at 500C. Lithium chloride has a decomposition potential of 3.684 v at
450C calculated from thermodynamic data, and the formal electrode potential for Li<sup>+1</sup> in eutectic LiCl-KCl at 450C is -3.41 v
measured against a Pt, Pt<sup>+2</sup> reference electrode. (See Fused Salt Electromotive Force Series.)
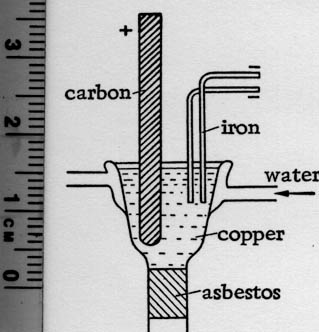
One type of cell used commercially is constructed of a covered steel pot suspended in a firebrick heating chamber, much like the magnesium cells. Gas
or oil is used to heat the cell. Top entering graphite anodes dip into the fused salt bath, and the steel cathodes are so proportioned that the
lithium rising from them to the surface of the bath is prevented from approaching the chlorine gas rising from the anode. Separate collecting means
are used for withdraw of the lithium and the chlorine. The molten lithium is carefully protected from contact with air, and is withdrawn from a
collecting vessel and cast into molds.
Another type of cell described recently<sup>1</sup> is designed like the Downs sodium cell. It is a covered, brick-lined cylindrical pot
26 in. in diameter and 30 in. deep with a single bottom entering anode 6 to 8 in. in diameter centered vertically in the pot. A steel cylindrical
cathode 9 to 12 in. inside diameter and 8 to 12 in. high surrounds part of the anode, and between them is a cylindrical diagram of perforated
24-gague, 316 stainless steel sheet, which keeps the chlorine rising along the anode from mixing with the lithium rising along the inside of the
cathode. A vertical hoodlike nickel chlorine collector is positioned over the top of the anode, and a steel collector placed over the cathode is used
to hold and guide the molten lithium to a vertical takeoff line continuously into a stainless steel holding tank. Auxiliary electric heating elements
in the bath maintain the temperature.
The lithium cells hold only a few hundred pounds of bath, and the production rate from cells using 900 to 1000 amperes is in the range of 8 to 10 lb
of lithium per day per cell.
Some typical operating data for commercial cells are given in table 1.
The cell described in Ref. 1 operates at somewhat lower voltage, current density, current efficiency, and unit energy consumption, and its chemical
efficiency is somewhat higher. While designed to operate at 1000 amperes, it can be operated up to 3500 amperes with no abnormal behavior.
Table 1. Lithium Cells-Operating Characteristics<sup>2</sup>
Current (amp) 850-900
Temperature
C 400-420
F 752-788
Voltage 8-9
Anodic c.d. (amp/sq. in.) 9.0
Cathodic c.d. (amp/sq. in.) 13.0
Current efficiency (%) 85-90
Unit energy (kwhr/lb) 18.2
Chemical consumption (LiCl/lb Li) 7.3
Chemical efficiency (%) 83.7
Cell capacity (lb) 220
Details about cell operation and about the physical properties and electrochemical behavior of lithium and its salts are presented in Refs. 1 and 2.
The separation of lithium isotopes 6 and 7 can be effected by the electrolysis in a mercury cathode cell and by electromigration techniques.
Information about both methods is given in the article. Isotopes-Electrochemical Separation; also see Electromigration in Liquid Metals.
References
1. Motock, G.T., Electrochem. Tech., 1, 122-127 (1963)
2. Landolt, P.E., and Sittig, M., chapter on "Lithium" in "Rare Metals Handbook," C.A. Hampel, Editor, New York, Reinhold Publishing Corp., 1961.
Clifford A. Hampel |


 so don't use your best one; perhaps you may have another method that is superior.
I freaked multiple times when when the battery warmed--due to me body heat and tight grip, combined with my tension! You may find a strip, one I
found was apparently Li but according to a reference there is another that isn't Li. Additionally, there are layers of Li and
MnO<sub>2</sub> in the large plastic roll. I used my pocket knife's scissors to cut through each layer of plastic after I had got
the Li on it. I do not know if one could cut open the entire battery and unroll the plastic film and then get a large layer of Li, but I may try it
when I next need Li for something (no use yet, so I expect it to be a LONG while considering my fright). Oh yeah, get used to all of the black
(supposedly red-brown) nitride that'll be all around when you're done.
so don't use your best one; perhaps you may have another method that is superior.
I freaked multiple times when when the battery warmed--due to me body heat and tight grip, combined with my tension! You may find a strip, one I
found was apparently Li but according to a reference there is another that isn't Li. Additionally, there are layers of Li and
MnO<sub>2</sub> in the large plastic roll. I used my pocket knife's scissors to cut through each layer of plastic after I had got
the Li on it. I do not know if one could cut open the entire battery and unroll the plastic film and then get a large layer of Li, but I may try it
when I next need Li for something (no use yet, so I expect it to be a LONG while considering my fright). Oh yeah, get used to all of the black
(supposedly red-brown) nitride that'll be all around when you're done. so I had to neutralize some carbonate with HCl so this is going to
take awhile, stupid hygroscopic/disquecent LiCl, it's boiling away right now though.
so I had to neutralize some carbonate with HCl so this is going to
take awhile, stupid hygroscopic/disquecent LiCl, it's boiling away right now though.
 ), and it started sizzling and boiling on the surface, lithium metal had been achieved
as a non-adherent black coating. Holding the test tube up to the sun I noticed flecks throughout the solution, "Lithium?" I thought to
myself.
), and it started sizzling and boiling on the surface, lithium metal had been achieved
as a non-adherent black coating. Holding the test tube up to the sun I noticed flecks throughout the solution, "Lithium?" I thought to
myself.




 . So there was no confirmatory aspect to it.
. So there was no confirmatory aspect to it. (yes 100€/100kg :cool
(yes 100€/100kg :cool
