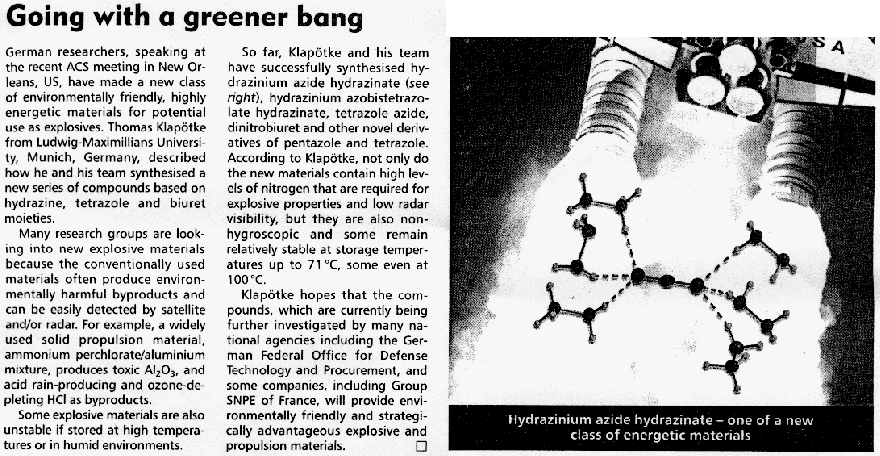
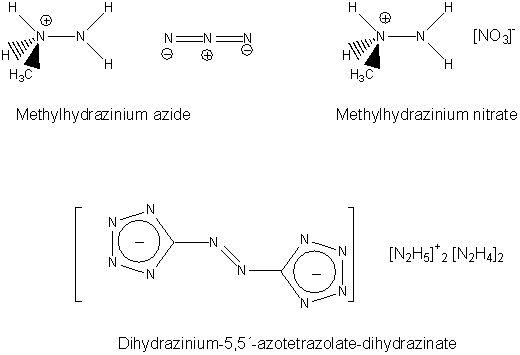
Next-generation Explosives: More Power And Safety Without The Pollution
ScienceDaily (May 27, 2008) — Scientists in Germany are reporting development of a new generation of explosives that is more powerful than TNT and
other existing explosives, less apt to detonate accidentally, and produce fewer toxic byproducts.
Their study of these more environmentally friendly explosives is scheduled for the June 24 issue of ACS’ Chemistry of Materials, a bi-weekly
journal.
In the new study, Thomas M. Klapötke and Carles Miró Sabate point out that conventional explosives such as TNT, RDX and HMX, widely-used in military
weapons, are rich in carbon and tend to produce toxic gases upon ignition.
In addition to polluting the environment, these materials are also highly sensitive to physical shock, such as hard impacts and electric sparks,
making their handling extremely dangerous. Greener, safer explosives are needed, the researchers say.
To meet this need, Klapötke and Sabate turned to a recently explored class of materials called tetrazoles, which derive most of their explosive
energy from nitrogen instead of carbon. They identified two promising tetrazoles: HBT and G2ZT. The researchers
developed tiny “bombs” out of these materials and detonated them in the laboratory. The materials showed less sensitivity to shock than
conventional explosives and produced fewer toxic products when burned, the researchers say.
--------------------------------------------------------------------------------
Journal reference:
Klapötke, Thomas M. and Sabaté, Carles Miró. Bistetrazoles: Nitrogen-Rich, High-Performing, Insensitive Energetic Compounds. Chem. Mater., 2008
doi: 10.1021/cm703657k
|


 !!
!!











 Although there is always a chance
Although there is always a chance
 . Maybe a polymer based on <a
href="http://www.sciencemadness.org/scipics/axt/bis-nibgtn2.jpg">this</a> is more likely
. Maybe a polymer based on <a
href="http://www.sciencemadness.org/scipics/axt/bis-nibgtn2.jpg">this</a> is more likely  Ive found reference that nitromethane-glyoxal will at least go to the cyclic dinitroinositol, an excess of NM was used
to achieve this.
Ive found reference that nitromethane-glyoxal will at least go to the cyclic dinitroinositol, an excess of NM was used
to achieve this. The problem however, is that most people do not want to use
their precious nitric acid to make glyoxal. However, the air oxidation of ethylene glycol seems very promising- it was how I originally figured I
would make glyoxal. After finding no mention of this process I decided it might not be worth it. And since formaldehyde has already been made in the
home lab like this it is probably the easiest method, IMHO. Of course, buying it is even better. I'll have to look into that and see how it
goes.
The problem however, is that most people do not want to use
their precious nitric acid to make glyoxal. However, the air oxidation of ethylene glycol seems very promising- it was how I originally figured I
would make glyoxal. After finding no mention of this process I decided it might not be worth it. And since formaldehyde has already been made in the
home lab like this it is probably the easiest method, IMHO. Of course, buying it is even better. I'll have to look into that and see how it
goes. (but
feel free to PM if you need something specific, I'll try to get it for you)
(but
feel free to PM if you need something specific, I'll try to get it for you)
 Must be more dense then it looks (though they dont define "energy". Its a simple reaction but crappy yields of dinitroinositol from NM +
glyoxal.
Must be more dense then it looks (though they dont define "energy". Its a simple reaction but crappy yields of dinitroinositol from NM +
glyoxal. [img]http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=72916[/img]
[img]http://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?t=l&cid=72916[/img] , no none that I know of , bear
, no none that I know of , bear  , 150.31 (2C, C-NH2); Decomposition Experiments. MS (EI): m/z = 12 (0.6, C4+), 13 (0.3,
CH3+), 14 (3.4, CH22+, N+), 15 (0.4, CH3+), 16 (10.0, CH4.+, NH2+), 17 (13.6, NH3.+), 26 (5.8, CN+), 27 (38.0, HCN.+), 28 (100.0, N2.+), 31 (0.1,
NH2NH+), 32 (0.2, NH2NH2.+), 38 (0.0, CCN3+), 39 (0.0, CHCN2+), 40 (0.1, CH2CN+), 41 (0.2, CH3CN.+). IR (Gas): Δν (cm−1) 3450 (w,
NH3), 3333 (s, HCN), 3285 (m, HCN), 2166 (w, HCN), 2102 (w, HCN), 1623 (s, NH3), 1431 (w, HCN), 1382 (w, HCN), 964 (vs, NH3), 925 (vs, NH3), 731 (m,
HCN), 714 (vs, HCN), 681 (m, HCN), 667 (w, NH3), 624 (vw, NH3).
, 150.31 (2C, C-NH2); Decomposition Experiments. MS (EI): m/z = 12 (0.6, C4+), 13 (0.3,
CH3+), 14 (3.4, CH22+, N+), 15 (0.4, CH3+), 16 (10.0, CH4.+, NH2+), 17 (13.6, NH3.+), 26 (5.8, CN+), 27 (38.0, HCN.+), 28 (100.0, N2.+), 31 (0.1,
NH2NH+), 32 (0.2, NH2NH2.+), 38 (0.0, CCN3+), 39 (0.0, CHCN2+), 40 (0.1, CH2CN+), 41 (0.2, CH3CN.+). IR (Gas): Δν (cm−1) 3450 (w,
NH3), 3333 (s, HCN), 3285 (m, HCN), 2166 (w, HCN), 2102 (w, HCN), 1623 (s, NH3), 1431 (w, HCN), 1382 (w, HCN), 964 (vs, NH3), 925 (vs, NH3), 731 (m,
HCN), 714 (vs, HCN), 681 (m, HCN), 667 (w, NH3), 624 (vw, NH3). That's life.
That's life.








