madmanhere - 21-2-2012 at 19:33
Hi,
I came across this website yesterday after reading some comments on YouTube. Awesome website this is!
Can you tell what would be the products of electrolysis of 0.5% w/w Sodium Hypochlorite solution using carbon electrodes?
Thanks.
Pulverulescent - 21-2-2012 at 23:54
O<sub>2</sub> and H<sub>2</sub>; decomposition of water, basically.
It's highly unlikely that Cl<sub>2</sub> would be evolved in such dilute soln.
P
woelen - 22-2-2012 at 01:32
Well, things will be more complicated. You will get all kinds of rather complex reactions at the anode, which lead to formation of chlorate ion,
chloride and oxygen. At the cathode you will get some hydrogen, but almost certainly there will also be reduction of hypochlorite to chloride ion:
ClO(-) + H2O + 2e --> Cl(-) + 2OH(-)
In fact, this latter reaction may even be the dominant reaction. It is a great problem in electrolytic production of chlorate salts from chlorides.
The addition of a small quantity of hexavalent chromium to the solution largely prevents the occurrence of this reaction and then indeed hydrogen gas
and hydroxide ions are produced at the cathode.
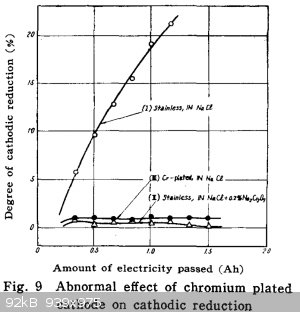
yobbo II - 20-9-2020 at 13:22
Could not find the post I was looking for where Woelen posted a link to his page showing cathodic reduction in action as hydrogen production from a
chlorate cell.
Some papers attached for anyone wanting some reading.
It would appear that you can substitute a chromium (plated) cathode for chromate addition to a cell to almost eliminate reduction of hypochlorite at
the cathode.
See denki kajuka for some graphs. No english translation.
Attachment: reduction.rar (2.1MB)
This file has been downloaded 266 times