<em><strong>Appendix 8</strong></em>
<div align="right"><em><strong>Purification and Decontamination of Mercury</strong></em></div>
Dirty mercury contains
dissolved metals, as well as organic materials, grease, oxides, and just plain dirt, which are usually found on top and in pockets trapped under the
surface.
The three steps in the purification process are: filtration, to remove grease, dirt, and
some dissolved metal; oxidation, to remove base metals; and distillation, to remove noble metals.
First, pass the mercury through a pinhole in a cone or funnel of filter paper or through
glass wool to remove dirt and some dissolved metal. The heavy mercury falls through the pinhole, while dirt, oxide, and grease will stick to the
paper and remain behind. As the mercury falls through the pinhole the surface layer in which metallic impurities are concentrated will contract. The
impurities will concentrate further in the contracting surface and lower the surface tension so that the impure surface mercury does not pass through
the pinhole. Each passage of mercury through a pinhole is equivalent to partial extraction. A dozen or so repeated filtrations will purify mercury
sufficiently for most purposes. However, it is usually better to filter once or twice and then to remove most active metals by oxidation.
<table><tr><td> This is done by forcing air through the mercury. The
simplest apparatus is shown in Diagram I. Put a layer of 10–20% nitric acid over the dirty mercury and pull air through by an aspirator or
vacuum line. The air will oxidize the base metal such as zinc, lead, etc., while stirring the mercury. The oxides will float to the top of the
mercury and dissolve in the acid solution. The nitric acid will also oxidize any base metal at the mercury surface. The bubble rate should be fast
enough to stir the mercury without splashing it into the side arm. The glass wool plug is present to protect the aspirator or vacuum line from acid
droplets. Usually 8 hours of oxidation at the rate of two to three air bubbles per second will clean about 10 pounds of mercury.
After the oxidation wash the mercury with distilled water. Then pass it through a pinhole
in a filter paper to remove the last traces of oxides. For most purposes the mercury will now be sufficiently clean. For example, the gold,
platinum, or silver still present will not affect routine density measurements or interfere with use in mercury contacts or seals.
When very pure mercury is require, as in a polarography and in surface tension
measurements, after the oxidation vacuum distill the mercury at 25–30 mm pressure. Use an all-Pyrex apparatus with a glass shield and
distill in a hood in case the apparatus breaks.</td><td>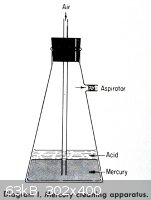 </td></tr></table> </td></tr></table>
<strong>REMOVAL OF SPILLED MERCURY</strong>
Spilled mercury on desk tops and in cracks in the floor is an <em>extremely
dangerous health hazard.</em> Mercury vapor is poisonous, and if inhales attacks the nails, hair, teeth, brain, and kidneys. To avoid
spillage, work with mercury over wide-mouth safety jars or pots. Place apparatus on trays so that if a container breaks and the mercury spills it
will be caught. To remove or deactivate spilled mercury, use sequestering agents, chemicals which lower the vapor pressure of the mercury and convert
it into a form that can be swept up. (Mercury splashes into droplets to small to be seen and too fluid to be swept up.)
Cover the contaminated areas with either sulfur or some other sequestering agent, such as
an organic sulfur compound. Sulfur, over an extended period of time, cuts down the vapor pressure by combining with the mercury to form solid mercury
sulfide, which may be swept up. Organic sequestering agents do not contain free sulfur but hydrolyze to form H<sub>2</sub>S, which then
reacts with the mercury. Dissolve the sequestering agent in water and wash the floors and desks with the solution. Vacate the room for a period of
time to avoid the hydrogen sulfide, which is an even more deadly poison than the mercury. After the room is ventilated, sweep up the mercury sulfide.
– <em>Laboratory Course in Physical Chemistry.</em> Salzberg, Morrow, & Cohen. Second Printing, Academic Press, 1966.
(<a href="http://books.google.com/books/about/Laboratory_course_in_physical_chemistry.html?id=jsc0AAAAMAAJ" target="_blank">Google
Books</a> <img src="../scipics/_ext.png" /> |




 ....
.... <sub>2</sub> solution, because at least
that would avoid any mercury vapour at all. Whether the formed mercury would coalesce into liquid mercury I don’t know (but I suspect it does).
Here’s one reference from the Journal of the American Chemical. Society that clearly suggests it is possible.
<sub>2</sub> solution, because at least
that would avoid any mercury vapour at all. Whether the formed mercury would coalesce into liquid mercury I don’t know (but I suspect it does).
Here’s one reference from the Journal of the American Chemical. Society that clearly suggests it is possible.


 . It would be worth trying this on a very small scale, using a long or extended test
tube, OUTSIDE and standing upwind from the experiment.
. It would be worth trying this on a very small scale, using a long or extended test
tube, OUTSIDE and standing upwind from the experiment.











 <sub>2</sub>Cr<sub>2</sub>O<sub>7</sub> sol. and then i used it as a cathode in NaHCO<sub>3</sub>
electrolite. I finnaly aded the mercury in distill water. It maked some effervessence, when the effervessence stoped the mercury was shining as a
mirror, in fact I can see me on the droplet. this is a modified brauer process, brauer use KMnO<sub>4</sub>.
<sub>2</sub>Cr<sub>2</sub>O<sub>7</sub> sol. and then i used it as a cathode in NaHCO<sub>3</sub>
electrolite. I finnaly aded the mercury in distill water. It maked some effervessence, when the effervessence stoped the mercury was shining as a
mirror, in fact I can see me on the droplet. this is a modified brauer process, brauer use KMnO<sub>4</sub>.









 <sub>2</sub>
<sub>2</sub>

