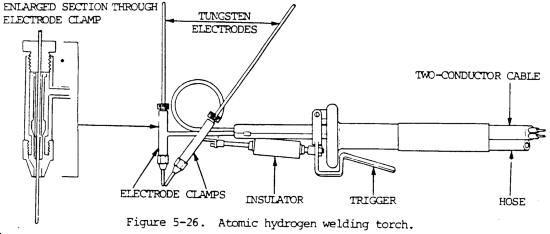
Atomic hydrogen welding (AHW) is an arc welding process that uses an arc between two metal tungsten electrodes in a shielding atmosphere of hydrogen.
The process was invented by Irving Langmuir in the course of his studies of atomic hydrogen. The electric arc efficiently breaks up the hydrogen
molecules, which later recombine with tremendous release of heat, reaching temperatures from 3400 to 4000 °C. Without the arc, an oxyhydrogen torch
can only reach 2800 °C.[1] This is the third hottest flame after cyanogen at 4525 °C and dicyanoacetylene at 4987 °C. An acetylene torch merely
reaches 3300 °C. This device may be called an atomic hydrogen torch, nascent hydrogen torch or Langmuir torch. The process was also known as arc-atom
welding.
The heat produced by this torch is sufficient to melt and weld tungsten (3422 °C), the most refractory metal. The presence of hydrogen also
acts as a gas shield and protects metals from contamination by carbon, nitrogen, or oxygen, which can
severely damage the properties of many metals. It eliminates the need of flux for this purpose.
The arc is maintained independently of the workpiece or parts being welded. The hydrogen gas is normally diatomic (H2), but where the temperatures are
over 600 °C (1100 °F) near the arc, the hydrogen breaks down into its atomic form, simultaneously absorbing a large amount of heat from the arc.
When the hydrogen strikes a relatively cold surface (i.e., the weld zone), it recombines into its diatomic form and rapidly releases the stored heat.
|













 Then dry, and
dessicate, recrystallize and dessicate again. Or I suppose I could just purchase it.
Then dry, and
dessicate, recrystallize and dessicate again. Or I suppose I could just purchase it.